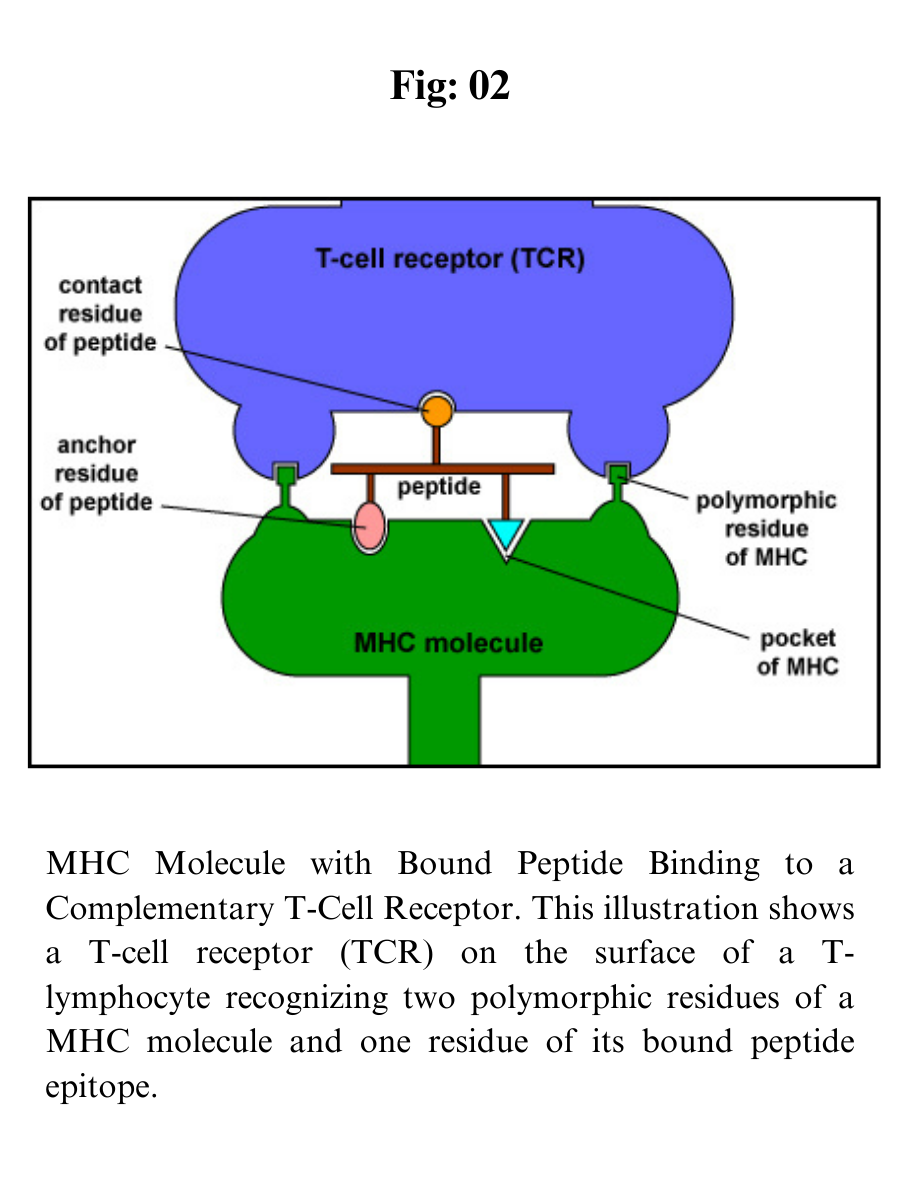
MHC molecules enable T-lymphocytes to recognize epitopes of antigens and discriminate self from non-self. Unlike B-cell receptors on B-lymphocytes that are able to directly bind epitopes on antigens, the T-cell receptors (TCRs) of T-lymphocytes can only recognize epitopes - typically short chains of amino acids called peptides - after they are bound to MHC molecules.
The MHC genes are the most polymorphic genes in the human genome, possessing many alleles for each gene.
The MHC genes are codominantly expressed so that an individual expresses the alleles inherited from each parent.
In this way, the number of MHC molecules that bind peptides for presentation to T-lymphocytes is maximized.
In addition, each MHC molecule is able to bind a wide variety of different peptides, both self-peptides and foreign peptides. There are two classes of MHC molecules: MHC-I and MHC-II.
MHC-I molecules present epitopes to T8-lymphocytes.
MHC-II molecules present epitopes to T4-lymphocytes.
The expression of MHC molecules is increased by cytokines produced during both innate immune responses and adaptive immune responses. Cytokines such as interferon-alpha, interferon-beta, interferon-gamma, tumor necrosis factor increase the expression of MHC-I molecules, while interferon-gamma is the main cytokine to increase the expression of MHC-II molecules.
MHC-I molecules
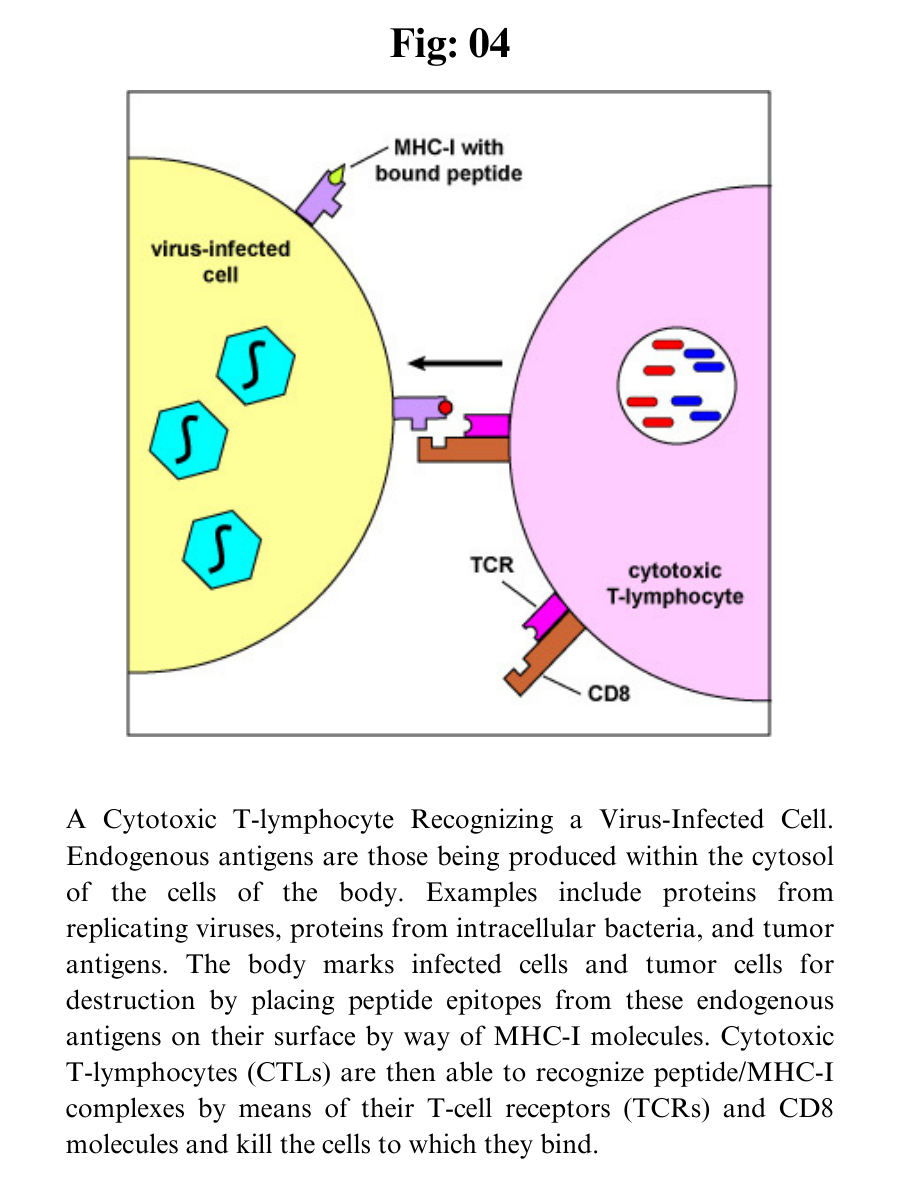
MHC-I molecules are designed to enable the body to recognize infected cells and tumor cells and destroy them with cytotoxic T-lymphocytes or CTLs.
CTLs are effector defense cells derived from naive T8-lymphocytes. MHC-I molecules are:
1. Made by all nucleated cells in the body.
2. Possess a deep groove that can bind peptide epitopes, typically 8-11 amino acids long, typically from endogenous antigens .
3. Present MHC-I/peptide complexes to naive T8-lymphocytes and cytotoxic T-lymphocytes possessing a complementary-shaped T-cell receptor or TCR.
4. Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of exogenous antigens to MHC-I molecules for eventual presentation to naive T8-lymphocytes.
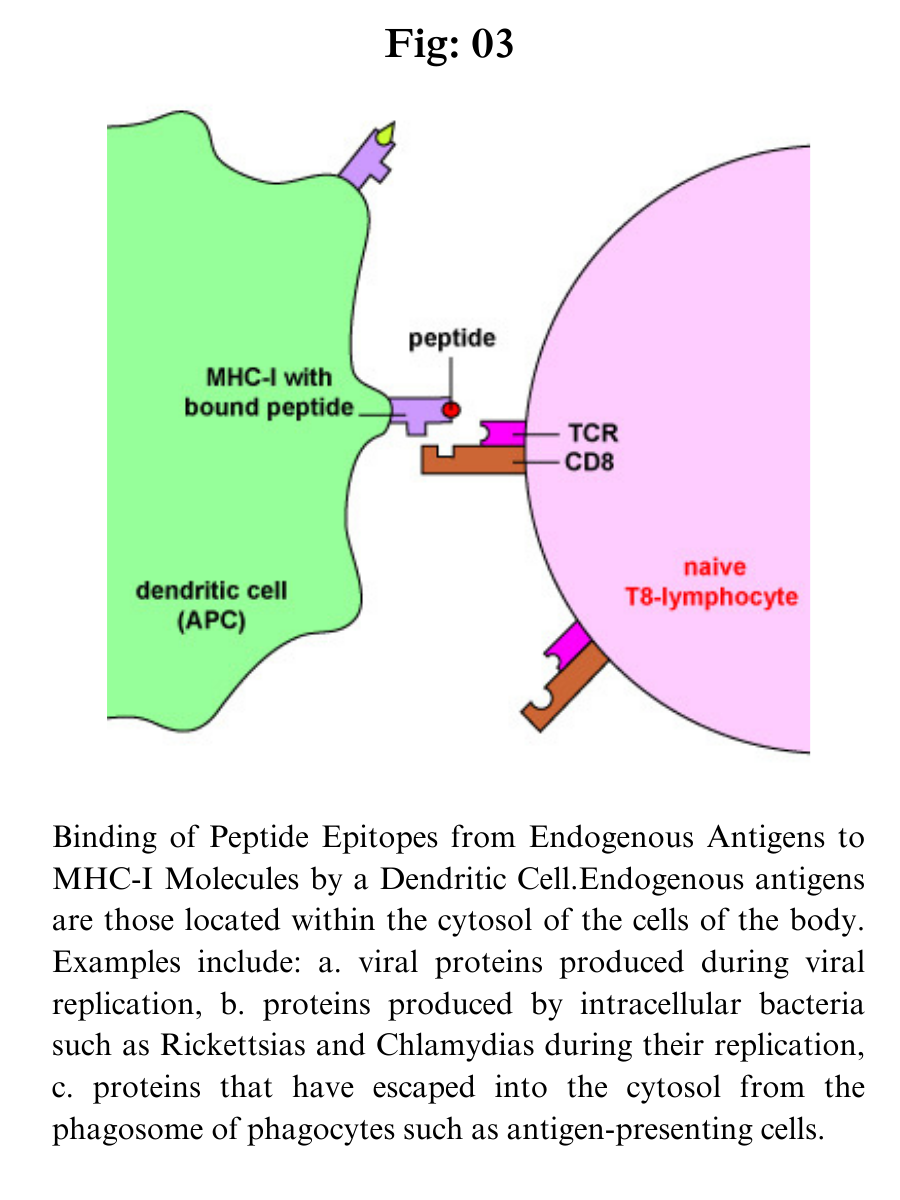
Endogenous antigens are proteins found within the cytosol of human cells. Examples of endogenous antigens include:
a. Viral proteins produced during viral replication;
b. Proteins produced by intracellular bacteria such as Rickettsias and Chlamydias during their replication;
c. Proteins that have escaped into the cytosol from the phagosome of phagocytes such as antigen-presenting cells;
d. Tumor antigens produced by cancer cells; and
e. Self-peptides from host cellular proteins.
During the replication of viruses and intracellular bacteria within their host cell, as well as during the replication of tumor cells, viral, bacterial, or tumor proteins are degraded into a variety of peptide epitopes by cylindrical organelles called proteasomes. The body's own cytosolic proteins are also degraded into peptides by proteasomes.
These peptide epitopes are then attached to a groove of MHC-I molecules that are then transported to the surface of that cell where they can be recognized by a complementary-shaped T-cell receptor (TCR) and a CD8 molecule, a co-receptor, on the surface of either a naive T8-lymphocyte or a cytotoxic T-lymphocyte (CTL).
The TCRs recognize both the foreign peptide antigen and the MHC molecule. TCRs, however, will not recognize self-peptides bound to MHC-I. As a result, normal cells are not attacked and killed.
Dendritic cells bind epitopes from endogenous antigens to MHC-I molecules and present them to naive T8-lymphocytes in order to activate these naive T8-lymphocytes.
a. Antigens are engulfed by dendritic cells and placed in a phagosome. Some of the proteins escape from the phagosome into the cytosol of the dendritic cell where they become endogenous antigens.
b. These endogenous antigens pass through proteasomes where they are degraded into a series of peptides.
c. The peptides are transported into the rough endoplasmic reticulum (ER) by a transporter protein called TAP.
d. The peptides then bind to the grooves of newly synthesized MHC-I molecules.
e. The endoplasmic reticulum transports the MHC-I molecules with bound peptides to the Golgi complex.
f. The Golgi complex, in turn, transports the MHC-I/peptide complexes by way of an exocytic vesicle to the cytoplasmic membrane where they become anchored. Here, the peptide and MHC-I/peptide complexes can be recognized by naive T8-lymphocytes by way of TCRs and CD8 molecules having a complementary shape.
Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of exogenous antigens to MHC-I molecules for eventual presentation to naive T8-lymphocytes.
MHC-I molecules with bound peptides on the surface of antigen-presenting dendritic cells can be recognized by a complementary-shaped TCR/CD8 on the surface of a naive T8-lymphocyte to initiate cell-mediated immunity. (Certain dendritic cells, as discussed later, can also cross-present exogenous antigens to MHC-I molecules).
MHC-I molecules with bound peptides on the surface of infected cells and tumor cells can be recognized by a complementary-shaped TCR/CD8 on the surface of a cytotoxic T-lymphocyte or CTL to initiate destruction of the cell containing the endogenous antigen. (CTLs are effector cells derived from naive T8-lymphocytes.)
Cytotoxic T-lymphocytes (CTLs) are then able to recognize peptide/MHC-I complexes by means of their T-cell receptors (TCRs) and CD8 molecules and kill the cells to which they bind.
a. During viral replication within the host cell, endogenous antigens, such as viral proteins, pass through proteasomes where they are degraded into a series of peptides.
b. The peptides are transported into the rough endoplasmic reticulum (ER) by a transporter protein called TAP.
c. The peptides then bind to the grooves of newly synthesized MHC-I molecules.
d. The endoplasmic reticulum transports the MHC-I molecules with bound peptides to the Golgi complex.
e. The Golgi complex, in turn, transports the MHC-I/peptide complexes by way of an exocytic vesicle to the cytoplasmic membrane where they become anchored. Here, the peptide and MHC-I/peptide complexes can be recognized by CTLs by way of TCRs and CD8 molecules having a complementary shape.
MHC-I molecules are coded for by three MHC-I genes, HLA-A, HLA-B, and HLA-C. As mentioned above, however, there are many different alleles for each gene that a person inherits. In this way, the number of MHC-I molecules that bind peptides for presentation to T-8 lymphocytes is maximized. The expression of MHC-I molecules on all cell types is increased by the cytokines interferon-alpha (IFN-a) and interferon-beta (IFN-ß).
MHC-II molecules
MHC-II molecules are designed to enable T4-lymphocytes to recognize epitopes of exogenous antigens and discriminate self from non-self. MHC-II molecules are:
Made by antigen-presenting cells or APCs, such as dendritic cells, macrophages, and B-lymphocytes.
Possess a deep groove that can bind peptide epitopes, often 10-30 amino acids long but with an optimum length of 12-16 amino acids, typically from exogenous antigens. The peptides interact along their entire length with the groove.
Present MHC-II/peptide complexes to naive T4-lymphocytes or effector T4-lymphocytes that have a complementary shaped T-cell receptor or TCR.
Through the process of cross-presentation, some antigen-presenting dendritic cells can cross-present epitopes of endogenous antigens to MHC-II molecules for eventual presentation to naive T4-lymphocytes.
Exogenous antigens are antigens that enter from outside the body, such as bacteria, fungi, protozoa, and free viruses. These exogenous antigens enter macrophages, dendritic cells, and B-lymphocytes through phagocytosis.
The microbes are engulfed and placed in a phagosome which then fuses with lysosomes. Following this fusion, the phagolysosome becomes acidified.
Acidification, in turn, activates the proteases within the phagolysosome enabling protein antigens from the microbe to be degraded into a series of short peptides.
These peptide epitopes are then attached to MHC-II molecules and are then transported to the surface of the antigen-presenting cell (APC). (Certain dendritic cells, as discussed later, can also cross-present endogenous antigens to MHC-II molecules.)
Some pathogens, such as Mycobacterium tuberculosis, Mycobacterium leprae, and Leishmania, are able to grow in the endocytic vesicles of macrophages without being killed by lysosomes. These macrophages can, however, become activated by T4-effector lymphocytes called TH1 cells and subsequently use intravesicular proteases to degrade the proteins from these pathogens into peptides for presentation to MHC-II molecules that pass through on their way to the cell surface.
Here the MHC-II molecules with bound peptides can be recognized by a complementary-shaped T-cell receptor and a CD4 molecule, a co-receptor, on the surface of a T4-lymphocyte. T4-lymphocytes are the cells the body uses to regulate both humoral immunity and cell-mediated immunity.
MHC-II molecules are coded for by three MHC-II genes, HLA-DR, HLA-DP, and HLA-DQ. Interferon-gamma (IFN- ?) increases the expression of both MHC-I and MHC-II molecules.