Introduction
Cell Viability is a common technique used by biochemists who are studying oncology and pharmaceutics. The most common use for cell viability studies is when determining the IC50 for a cytotoxic compound in cell culture. However, as you can expect, there are a lot of different times when you need to know if your cells are alive.
In larger pharmaceutical companies, MTT Cell Viability studies for Cytotoxic compounds are performed as a high throughput method because companies routinely screen MASSIVE libraries of small molecule drugs. To measure cell viability, researchers typically use an MTT assay, Cell Titer Blue, Trypan blue exclusion, or ATP assay.
In this method guide, we will walk through the theory behind all these methods and then end with a protocol for the MTT assay. It would be a great test of your skills if you could use our High Performance Liquid Chromatography (HPLC) Method Guide to detect the products of the MTT assay.
Using an MTT Assay to measure Cytotoxicity
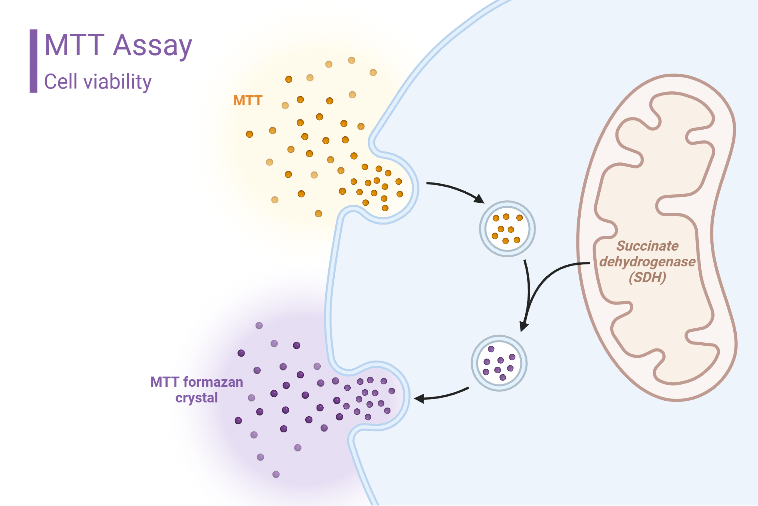
In general, to measure cell viability, you need to incubate cells with a reagent and measure the conversion of your reagent into a product. If lots of cells are alive, most of your reagent will be converted. If lots of cells are dead, then your reagent will only be partially converted. For the MTT assay, the reagent used is
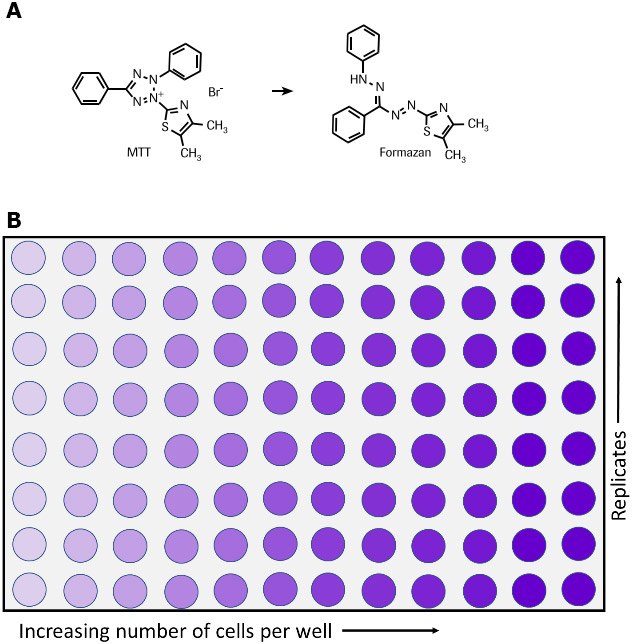
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium.
This is a positively charged small molecule that undergoes NADPH-mediated conversion over to Formazan. Because of its positive charge, MTT can enter viable cells and non-viable cells with ease.
Upon conversion, the Formazan product precipitates inside cells near the cell surface and can be detected using a spectrophotometer. Note: MTT only needs an intact and functioning mitochondria to be converted so it is a metabolic assay and not a proliferation assay. I’ll discuss cell proliferation assays in the future. The assay technique is very simple:
1.Grow an equal number of cells in different wells of a microplate
2.Add your cytotoxic compound and incubate
3.Then replace the media and add the MTT, let the cells convert the MTT (blue) into Formazan (purple)
4.Use SDS along with DMF or DMSO to resolubilize the formazan and to kill cells (stop them from converting any more reagent)
5.Then measure how much formazan was created using a spectrophotometer.
Protocol for Cell Viability MTT Assay
Materials for MTT Assay
MTT Solution (5 mg/ml MTT in PBS, pH 7.4, #M2128 Sigma Aldrich)
Solubilization solution, recipe here:
40% v/v Dimethylformamide #D4551 Sigma Aldrich
2% Glacial Acetic Acid #320099 Sigma Aldrich
16% Sodium Dodecyl Sulfate #436143 Sigma Aldrich
pH 4.7 & 37oC
96 well plate
Hep G2 cells
Complete DMEM (indicator-free, no phenol-red) with 10% Fetal Bovine Serum
PBS
Cytotoxic compound (ex: Doxorubicin)
Step-by-Step Cell Viability MTT Assay
Make the above solutions. Store MTT solution protected from light at 4oC and make sure there is no precipitate in the Solubilization solution.
Seed 25 x 103 Hep G2 cells in a 96 well plate with 250 ul of DMEM.
Add your cytotoxic compound (5 uM for Doxorubicin). Incubate for a desired time period (24 hours for Doxorubicin).
Aspirate media and wash 3x with PBS.
Add 125 ul of DMEM with 25 ul of MTT Solution. Incubate for 2 hours at 37oC.
Add in 100 ul of solubilization solution.
Pipette gently to mix without creating bubbles.
Measure via absorbance at 570 nm using spectrophotometer.
MTT Assay Notes, Tips, and Tricks
Always set up positive and negative controls! For positive controls have cells untreated with any cytotoxic compound as part of your wells. For negative controls have cells treated with 3% SDS as part of your wells. Also, make sure to have wells that have no cells, only media.
Increasing the number of cells also increases your signal
Too much MTT forms Formazan crystals which will damage cells so you might see the cells changing morphology.
This is an end-point assay because the precipitate inside the cells will kill them. Don’t plan on keeping your cells alive for any further studies after you add the MTT.
Having thiol-containing compounds in solution will convert MTT over to Formazan, so you’ll get false-positive data.
Having phenol-red in your medium may also convolute your results. Dye-free media is important to use.