Monoclonal Antibodies (mAb) Production
Introduction
Monoclonal antibody, antibody produced artificially through genetic engineering and related techniques. Production of monoclonal antibodies was one of the most important techniques of biotechnology to emerge during the last quarter of the 20th century. When activated by an antigen, a circulating B cell multiplies to form a clone of plasma cells, each secreting identical immunoglobulin molecules. It is such immunoglobulins—derived from the descendants of a single B cell—that are called monoclonal antibodies.
The antibody response to a natural infection or an active immunization, however, is polyclonal. In other words, it involves many B cells, each of which recognizes a different antigenic determinant (epitope) of the immunizing antigen and secretes a different immunoglobulin.
Thus the blood serum of an immunized person or animal normally contains a mixture of antibodies, all capable of combining with the same antigen but with different epitopes that appear on the surface of the antigen.
Furthermore, even antibodies that bind to the same epitope often have different abilities to bind to that epitope. This makes isolating an appreciable quantity of a particular monoclonal antibody from the polyclonal mixture extremely difficult.
Hybridoma
An astonishingly high serum concentration of a single type of immunoglobulin is associated with multiple myeloma, a type of cancer in which a single B cell proliferates to form a tumorous clone of antibody-secreting cells that can multiply indefinitely, like all cancer cells (see immune system disorder: Cancers of the lymphocytes).
Thus the immunoglobulins made by myelomas are monoclonal, and myeloma cells have been propagated to produce large quantities of monoclonal antibodies, which have been used to study the basic nature of immunoglobulins. Unfortunately, however, the antigen to which the myeloma antibodies bind is unknown.
If an immunologist wanted to obtain large amounts of a particular antibody—say, the anti-Rh antibody—the induction of myelomas is useless, for it has proved impossible to specify beforehand what antibody will be secreted by any given myeloma.
However, it is possible to produce large amounts of a chosen, identifiable monoclonal antibody (see illustration). Occasionally a cultured myeloma cell line continues to grow well but loses its ability to secrete immunoglobulin.
In 1975 the immunologists Georges Köhler and César Milstein fused non-antibody-secreting cultured myeloma cells with normal B cells from the spleen of an immunized mouse.
The fusion of a myeloma cell from a line that has lost the ability to secrete immunoglobulin with a B cell known to secrete a particular antibody results in a remarkable hybrid cell that produces the antibody made by its B-cell component but retains the capacity of its myeloma component to multiply indefinitely. Such a hybrid cell is called a hybridoma.
Because of hybridomas, researchers can obtain monoclonal antibodies that recognize individual antigenic sites on almost any molecule, from drugs and hormones to microbial antigens and cell receptors.
The exquisite specificity of monoclonal antibodies and their availability in quantity have made it possible to devise sensitive assays for an enormous range of biologically important substances and to distinguish cells from one another by identifying previously unknown marker molecules on their surfaces.
For example, monoclonal antibodies that react with cancer antigens can be used to identify cancer cells in tissue samples. Moreover, if short-lived radioactive atoms are added to these antibodies and they are then administered in tiny quantities to a patient, they become attached exclusively to the cancer tissue.
By means of instruments that detect the radioactivity, physicians can locate the cancerous sites without surgical intervention. Monoclonal antibodies also have been used experimentally to deliver cytotoxic drugs or radiation to cancer cells.
Human monoclonal antibodies
Although the preparation of monoclonal antibodies from rat or mouse cells has become routine practice, the construction of human hybridomas has not been as easy. This is partly because most human myeloma cells do not grow well in culture, and those that do have not produced stable hybridomas.
If, however, human B cells isolated from blood are infected by the Epstein-Barr virus (the agent that causes infectious mononucleosis), they can be propagated in culture, where they continue to secrete immunoglobulin. Very few of them are likely to produce an antibody with a desired specificity, even from a subject who has been immunized; but in some instances immunologists have succeeded in identifying and selecting cells that secrete the wanted immunoglobulin.
These cells can be grown in culture as single clones that secrete a monoclonal antibody. Researchers have used this process to obtain human monoclonal antibodies against the Rh antigen.
Producing Monoclonal Antibodies
This method for production of monoclonal antibodies is called hybridoma technology. Since then, monoclonal antibodies have been widely used as an essential tool of biomedical research and therapeutic applications.
Another method of manufacturing monoclonal antibodies is by using phage display which was discovered by G. Smith in 1985. And it has become one of the most effective techniques for producing large amounts of peptides, proteins and antibodies.
Based on hybridoma and phage display technology, Sino Biological has the capability to offer custom monoclonal antibody services as well as large scale antibody production and purification. Whatever your specifications are, Sino Biological can propose tailored solutions and its expertise to successfully complete each project.
Steps to Produce Monoclonal Antibodies in Mouse
Some assays require better antibody specificity and affinity than can be obtained using a polyclonal antiserum. To achieve this high specificity, all of the antibodies must bind with high affinity to a single epitope. This high specificity can be provided by monoclonal antibodies (mAbs). Production of monoclonal antibodies involves several critical procedures as follows.
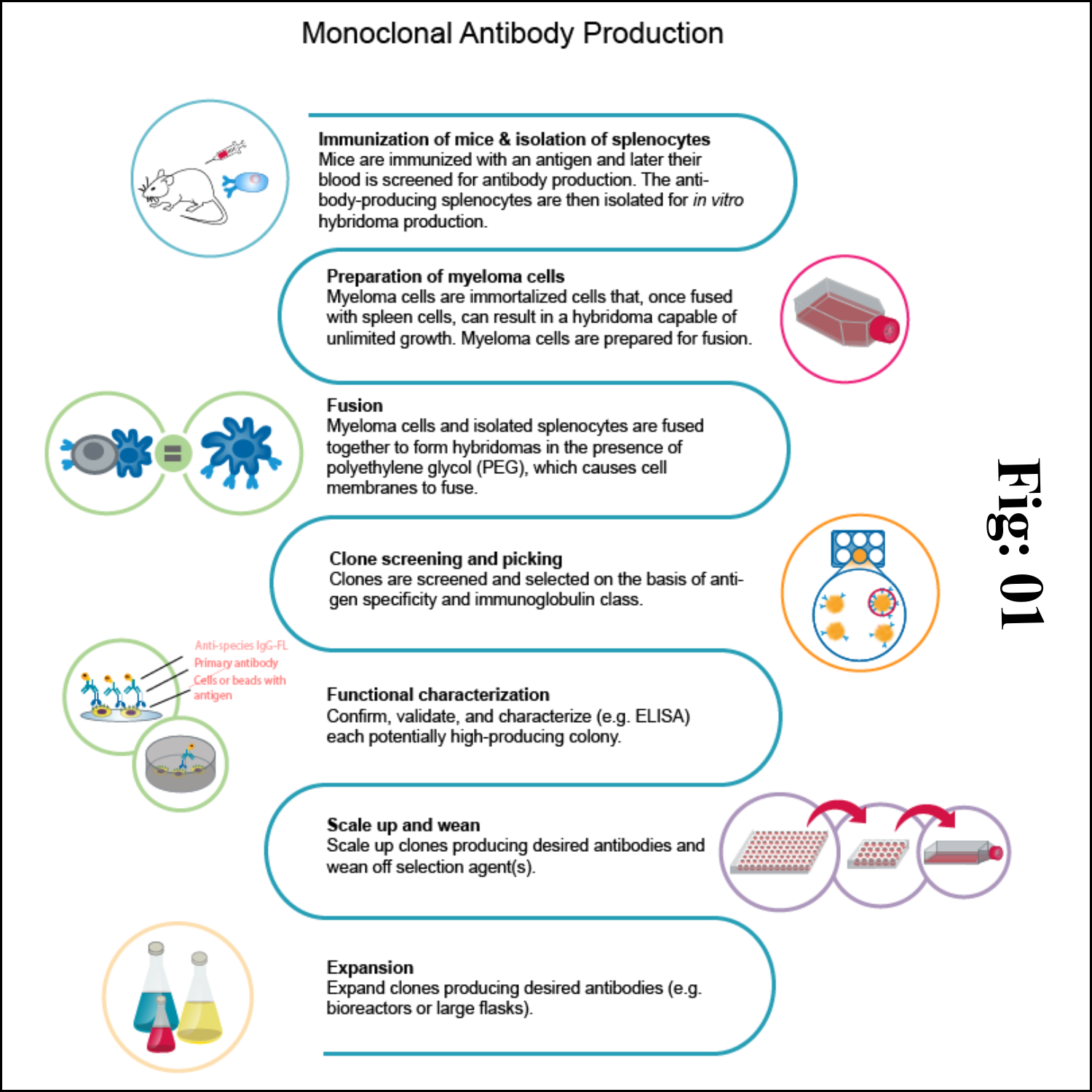
1) Animal immunization
The very first step in hybridoma technology is to immunize an animal (usually a mouse), with appropriate antigen. The antigen, along with an adjuvant like Freund's complete or incomplete adjuvant is injected subcutaneously (adjuvants are non-specific potentiators of specific immune responses). The injections at multiple sites are repeated several times.
This enables increased stimulation of B-lymphocytes which are responding to the antigen. Three days prior to killing of the animal, a final dose of antigen is intravenously administered. The immune-stimulated cells for synthesis of antibodies have grown maximally by this approach. The concentration of the desired antibodies is assayed in the serum of the animal at frequent intervals during the course of immunization.
When the serum concentration of the antibodies is optimal, the animal is sacrificed. The spleen is aseptically removed and disrupted by mechanical or enzymatic methods to release the cells. The lymphocytes of the spleen are separated from the rest of the cells by density gradient centrifugation.
2) Fusion & Selection
The thoroughly washed lymphocytes are mixed with HGPRT defective myeloma cells. The mixture of cells is exposed to polyethylene glycol (PEG) for a short period (a few minutes), since it is toxic. PEG is removed by washing and the cells are kept in a fresh medium. These cells are composed of a mixture of hybridomas (fused cells), free myeloma cells and free lymphocytes.
When the cells are cultured in HAT medium, only the hybridoma cells grow, while the rest will slowly disappear. This happens in 7-10 days of culture. Selection of a single antibody producing hybrid cells is very important. This is possible if the hybridomas are isolated and grown individually. The suspension of hybridoma cells is so diluted that the individual aliquots contain on an average one cell each. These cells, when grown in a regular culture medium, produce the desired antibody.
3) Antibody purification
Monoclonal antibodies may need to be purified before they are used for a variety of purposes. Protein A affinity chromatography is used to purify monoclonal antibodies. It's the golden standard for monoclonal antibody (mAb) purification, and a technology that has gained high interest because of its great performance and capabilities. If customers need higher purity, further purification methods can be performed.
4) Quality control
After purification, a series of quality control tests are performed to ensure the quality of monoclonal antibodies. Antibody concentration is evaluated by absorption at 280 nm (A280). The purity of monoclonal antibodies is checked using SDS-PAGE. To estimate the monoclonal antibody titer, an ELISA test was the most suitable method.
Application of Monoclonal Antibodies
Applications of monoclonal antibodies (mAbs) have become even broader in the past few years, as availability of highest-quality immunoglobulins and IgG-derivatives increased while cost-efficiency in manufacturing processes has progressed as well.
Diagnostic applications of monoclonal antibodies
Monoclonal antibodies are widely used in medical diagnostic tests. Due to their high affinity and high selectivity, they are applied as reagents and receptors in assays to detect their respective target antigen in the most demanding matrix: tissue and bodily fluids. Good examples of diagnostic application of monoclonal antibodies that everyone of us knows are the test for the detection of COVID-19 or the pregnancy test.
Examples of monoclonal antibodies in diagnostic applications:
1. Enzyme-linked immunosorbent assay (ELISA): color change in the presence of the antigen.
2. Western immunoblotting: molecular biology technique to detect and quantify proteins in complex mixtures.
3. Immunohistochemistry: fluorescently staining tissue sections to detect and map the distribution of cancer cells, e.g. characterization of colorectal cancer, breast cancer, etc.
4. Test kits: presence of a specific antigen (e.g. a biomarker for a disease, a determinant for disease severity, or fragments of pathogens in infectious diseases) leads to a visually recognizable feature, e.g. a coloured line on the inspection field of the test.
Therapeutic uses of monoclonal antibodies
Antibody therapy generally relies on the highest-quality monoclonal antibodies produced by biotechnology methods. Polyclonal antibodies are less expensive, but they are not specific to single epitopes and would lead to side effects. Moreover, they are produced in non-human animals and would lead to severe immunogenicity.
After the development of monoclonal antibody production by hybridoma technology by Köhler and Milstein in the 1970s, enhanced murine, chimeric, humanized and human antibodies were accessible for broader use. Subsequently, the first therapeutic monoclonal antibodies gained FDA approval after large clinical trials.
Monoclonal antibodies are particularly used for therapies in autoimmune diseases or cancer.
mAbs against autoimmune diseases
A class of diseases stems from a malfunction of the immune system, which then attacks the body’s own cells and its tissues. Several lines of treatments are routinely used to regulate immune responses, such as nonsteroidal antiinflammatory drugs, immunosuppressants and monoclonal antibodies.
Such mAbs’ pharmacology may be optimized at the Fab region to bind and inactivate certain pro-inflammatory peptides like cytokines or even specifically target the dysregulated immune cells.
Anti inflammatory mAbs that act as inhibitors of the physiologic TNF-response are routinely used in conditions such as Crohn’s disease, ulcerative colitis and rheumatoid arthritis. Monoclonal antibodies may be used as preventive treatments after organ transplants to avoid the development of the graft-versus-host-disease (GVHD), in which T lymphocytes (T cells) of the donated tissue recognize the host’s cells as foreign and begin immune responses.
Application of mAbs in cancer therapy
Monoclonal antibodies (mAbs) have revolutionized cancer therapy and are widely used in the treatment of various types of cancer. Here are some applications of mAbs in cancer therapy:
Targeted Therapy: Monoclonal antibodies are a type of targeted drug therapy that specifically recognize and bind to proteins on cancer cells. By targeting these specific proteins, mAbs can interfere with the growth and survival of cancer cells.
Immunotherapy: Some mAbs work by triggering the immune system to attack and kill cancer cells. They can attach themselves to cancer cells, making it easier for the immune system to identify and destroy them. This process is known as antibody-dependent cell-mediated cytotoxicity (ADCC).
Delivering Therapeutic Agents: Monoclonal antibodies can be conjugated with therapeutic agents such as chemotherapy drugs or radioactive particles. These conjugated mAbs act as homing devices, delivering the therapeutic agents directly to the cancer cells, thereby enhancing their effectiveness.
Blocking Cancer Cell Signals: Certain cancer cells have receptors that signal them to divide and multiply. Monoclonal antibodies can block these signals, preventing cancer cells from proliferating.
Inhibiting Immune Checkpoints: Immune checkpoints are molecules that regulate the immune response. Some monoclonal antibodies can inhibit these checkpoints, allowing the immune system to recognize and eliminate cancer cells more effectively.
The field of mAb research and development in cancer therapy is constantly evolving, with new mAbs being developed and tested for different types of cancer.
Further areas of application of monoclonal antibodies
Monoclonal antibodies are successfully used in therapies for various diseases, such as AIDS, asthma or COVID-19. Here’s a summary of their use in each of these areas:
AIDS: mAbs have been explored as a potential treatment for HIV/AIDS. They can target specific viral proteins and inhibit viral replication. However, the development of effective mAbs for HIV has been challenging due to the high mutation rate of the virus.
COVID-19: Monoclonal antibodies have emerged as a promising therapeutic approach for COVID-19. Several mAbs have been authorized for emergency use or received full approval for the treatment of COVID-19. They can neutralize the SARS-CoV-2 virus, reduce viral load, and potentially prevent severe disease progression.
Asthma: mAbs have shown promise in the treatment of asthma. They can target specific molecules involved in the inflammatory response, such as immunoglobulin E (IgE) or interleukins, to reduce airway inflammation and improve asthma control.
Viral Infections: Monoclonal antibodies have been investigated for their potential in combating various viral infections. They can directly neutralize viruses, enhance immune responses, and provide prophylactic benefits. mAbs have been studied for viruses such as Ebola, respiratory syncytial virus (RSV), cytomegalovirus (CMV), and others.
Advantages of monoclonal antibodies
• Same quality of the antibody is maintained amongst the different production batches.
• Highly reproducible and scalable, unlimited production source.
• Speed and sensitivity and specificity of assays.
• Can produce antibodies when needed.
• No need to worry about maintaining the animals.
• Antigen or immunogen need not be pure.
• Selection helps to identify the right clones against the specific antigen.