Introduction
Electron Transport Chain is a series of compounds where it makes use of electrons from electron carrier to develop a chemical gradient. It could be used to power oxidative phosphorylation. The molecules present in the chain comprises enzymes that are protein complex or proteins, peptides and much more.
Large amounts of ATP could be produced through a highly efficient method termed oxidative phosphorylation. ATP is a fundamental unit of metabolic process. The electrons are transferred from electron donor to the electron acceptor leading to the production of ATP. It is one of the vital phases in the electron transport chain. Compared to any other part of cellular respiration the large amount of ATP is produced in this phase.
Electron transport is defined as a series of redox reaction that is similar to the relay race. It is a part of aerobic respiration. It is the only phase in glucose metabolism that makes use of atmospheric oxygen.
When electrons are passed from one component to another until the end of the chain the electrons reduce molecular oxygen thus producing water. The requirement of oxygen in the final phase could be witnessed in the chemical reaction that involves the requirement of both oxygen and glucose.
The electron transport chain is a collection of membrane-embedded proteins and organic molecules, most of them organized into four large complexes labeled I to IV. In eukaryotes, many copies of these molecules are found in the inner mitochondrial membrane. In prokaryotes, the electron transport chain components are found in the plasma membrane.
As the electrons travel through the chain, they go from a higher to a lower energy level, moving from less electron-hungry to more electron-hungry molecules. Energy is released in these “downhill” electron transfers, and several of the protein complexes use the released energy to pump protons from the mitochondrial matrix to the intermembrane space, forming a proton gradient.
Electron Transport Chain in Mitochondria
A complex could be defined as a structure that comprises a weak protein, molecule or atom that is weakly connected to a protein. The plasma membrane of prokaryotes comprises multi copies of the electron transport chain.
Complex 1- NADH-Q oxidoreductase: It comprises enzymes consisting of iron-sulfur and FMN. Here two electrons are carried out to the first complex aboard NADH. FMN is derived from vitamin B2.
Q and Complex 2- Succinate-Q reductase: FADH2 that is not passed through complex 1 is received directly from complex 2. The first and the second complexes are connected to a third complex through compound ubiquinone (Q). The Q molecule is soluble in water and moves freely in the hydrophobic core of the membrane. In this phase, an electron is delivered directly to the electron protein chain. The number of ATP obtained at this stage is directly proportional to the number of protons that are pumped across the inner membrane of the mitochondria.
Complex 3- Cytochrome c reductase: The third complex is comprised of Fe-S protein, Cytochrome b, and Cytochrome c proteins. Cytochrome proteins consist of the heme group. Complex 3 is responsible for pumping protons across the membrane. It also passes electrons to the cytochrome c where it is transported to the 4th complex of enzymes and proteins. Here, Q is the electron donor and Cytochrome C is the electron acceptor.
Complex 4- Cytochrome c oxidase: The 4th complex is comprised of cytochrome c, a and a3. There are two heme groups where each of them is present in cytochromes c and a3. The cytochromes are responsible for holding oxygen molecule between copper and iron until the oxygen content is reduced completely. In this phase, the reduced oxygen picks two hydrogen ions from the surrounding environment to make water.
Oxidative Phosphorylation
The electron transport chain is a series of proteins and organic molecules found in the inner membrane of the mitochondria. Electrons are passed from one member of the transport chain to another in a series of redox reactions.
Energy released in these reactions is captured as a proton gradient, which is then used to make ATP in a process called chemiosmosis.
Together, the electron transport chain and chemiosmosis make up oxidative phosphorylation. The key steps of this process, shown in simplified form in the diagram above, include:
1.Delivery of electrons by NADH and FADH Reduced electron carriers (NADH and FADH2) from other steps of cellular respiration transfer their electrons to molecules near the beginning of the transport chain. In the process, they turn back into NAD +, end superscript and FAD, which can be reused in other steps of cellular respiration.
2.Electron transfer and proton pumping. As electrons are passed down the chain, they move from a higher to a lower energy level, releasing energy. Some of the energy is used to pump H+, end superscript ions, moving them out of the matrix and into the intermembrane space. This pumping establishes an electrochemical gradient.
3.Splitting of oxygen to form water. At the end of the electron transport chain, electrons are transferred to molecular oxygen, which splits in half and takes up H+, end superscript to form water.
4.Gradient-driven synthesis of ATP. As H +, end superscript ions flow down their gradient and back into the matrix, they pass through an enzyme called ATP synthase, which harnesses the flow of protons to synthesize ATP.
All of the electrons that enter the transport chain come from NADH and FADH 2 molecules produced during earlier stages of cellular respiration: glycolysis, pyruvate oxidation, and the citric acid cycle.
NADH is very good at donating electrons in redox reactions (that is, its electrons are at a high energy level), so it can transfer its electrons directly to complex I, turning back into NAD +. As electrons move through complex I in a series of redox reactions, energy is released, and the complex uses this energy to pump protons from the matrix into the intermembrane space.
FADH 2 is not as good at donating electrons as NADH (that is, its electrons are at a lower energy level), so it cannot transfer its electrons to complex I. Instead, it feeds them into the transport chain through complex II, which does not pump protons across the membrane.
It has two important functions:
1.Regenerates electron carriers. NADH and FADH 2 pass their electrons to the electron transport chain, turning back into NAD + and FAD. This is important because the oxidized forms of these electron carriers are used in glycolysis and the citric acid cycle and must be available to keep these processes running.
2.Makes a proton gradient. The transport chain builds a proton gradient across the inner mitochondrial membrane, with a higher concentration of H + in the intermembrane space and a lower concentration in the matrix. This gradient represents a stored form of energy, and, as we’ll see, it can be used to make ATP.
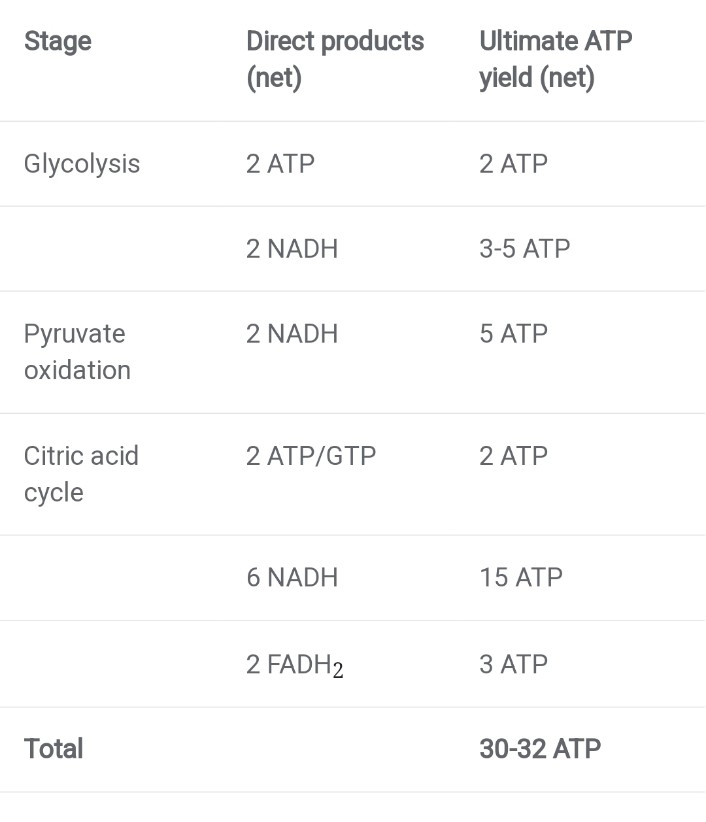
ATP yield
How many ATP do we get per glucose in cellular respiration? If you look in different books, or ask different professors, you'll probably get slightly different answers. However, most current sources estimate that the maximum ATP yield for a molecule of glucose is around 30-32 ATP. This range is lower than previous estimates because it accounts for the necessary transport of ADP into, and ATP out of, the mitochondrion.
Two net ATP are made in glycolysis, and another two ATP (or energetically equivalent GTP) are made in the citric acid cycle. Beyond those four, the remaining ATP all come from oxidative phosphorylation. Based on a lot of experimental work, it appears that four H + ions must flow back into the matrix through ATP synthase to power the synthesis of one ATP molecule. When electrons from NADH move through the transport chain, about 10 H + ions are pumped from the matrix to the intermembrane space, so each NADH yields about 2.5 ATP. Electrons from FADH 2, which enter the chain at a later stage, drive pumping of only 6 H +, leading to production of about 1.5 ATP.
One number in this table is still not precise, the ATP yield from NADH made in glycolysis. This is because glycolysis happens in the cytosol, and NADH can't cross the inner mitochondrial membrane to deliver its electrons to complex I. Instead, it must hand its electrons off to a molecular “shuttle system” that delivers them, through a series of steps, to the electron transport chain.
Some cells of your body have a shuttle system that delivers electrons to the transport chain via FADH 2. In this case, only 3 ATP are produced for the two NADH of glycolysis.
Other cells of your body have a shuttle system that delivers the electrons via NADH, resulting in the production of 5 ATP.
In bacteria, both glycolysis and the citric acid cycle happen in the cytosol, so no shuttle is needed and 5 ATP are produced.
30-32 ATP from the breakdown of one glucose molecule is a high-end estimate, and the real yield may be lower. For instance, some intermediates from cellular respiration may be siphoned off by the cell and used in other biosynthetic pathways, reducing the number of ATP produced.
Cellular respiration is a nexus for many different metabolic pathways in the cell, forming a network that’s larger than the glucose breakdown pathways alone.