Introduction
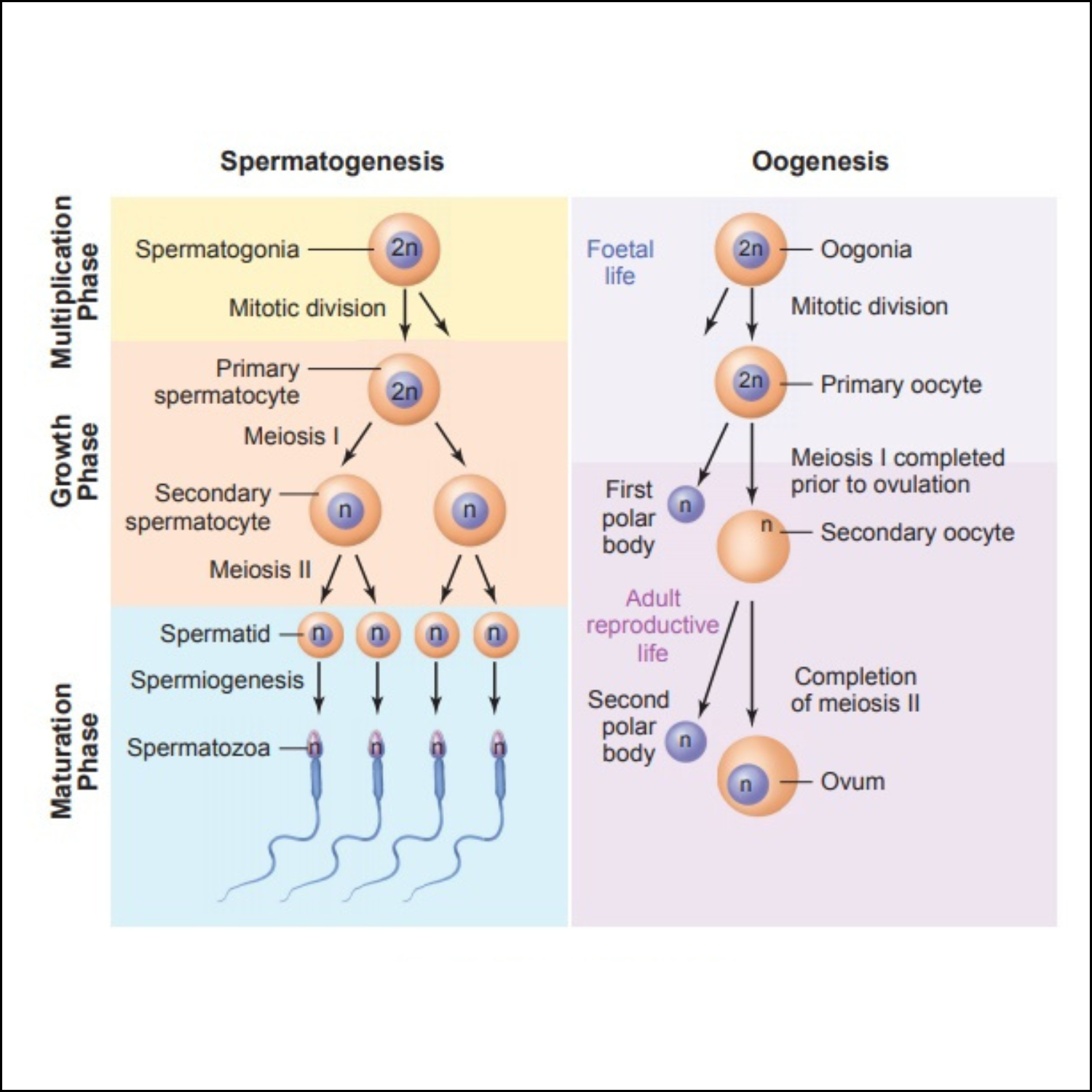
Oogenic meiosis
Oogenesis—the differentiation of the ovum—differs from spermatogenesis in several ways. Whereas the gamete formed by spermatogenesis is essentially a motile nucleus, the gamete formed by oogenesis contains all the materials needed to initiate and maintain metabolism and development.
Therefore, in addition to forming a haploid nucleus, oogenesis also builds up a store of cytoplasmic enzymes, mRNAs, organelles, and metabolic substrates. While the sperm becomes differentiated for motility, the egg develops a remarkably complex cytoplasm.
Mechanism
The mechanisms of oogenesis vary among species more than those of spermatogenesis. This difference should not be surprising, since patterns of reproduction vary so greatly among species. In some species, such as sea urchins and frogs, the female routinely produces hundreds or thousands of eggs at a time, whereas in other species, such as humans and most mammals, only a few eggs are produced during the lifetime of an individual.
In those species that produce thousands of ova, the oogonia are self-renewing stem cells that endure for the lifetime of the organism. In those species that produce fewer eggs, the oogonia divide to form a limited number of egg precursor cells.
In the human embryo, the thousand or so oogonia divide rapidly from the second to the seventh month of gestation to form roughly 7 million germ cells.
After the seventh month of embryonic development, however, the number of germ cells drops precipitously. Most oogonia die during this period, while the remaining oogonia enter the first meiotic division (Pinkerton et al. 1961).
These latter cells, called the primary oocytes, progress through the first meiotic prophase until the diplotene stage, at which point they are maintained until puberty. With the onset of adolescence, groups of oocytes periodically resume meiosis.
Thus, in the human female, the first part of meiosis begins in the embryo, and the signal to resume meiosis is not given until roughly 12 years later.
In fact, some oocytes are maintained in meiotic prophase for nearly 50 years. As indicated, primary oocytes continue to die even after birth. Of the millions of primary oocytes present at birth, only about 400 mature during a woman's lifetime.
Oogenic meiosis also differs from spermatogenic meiosis in its placement of the metaphase plate. When the primary oocyte divides, its nucleus, called the germinal vesicle, breaks down, and the metaphase spindle migrates to the periphery of the cell.
At telophase, one of the two daughter cells contains hardly any cytoplasm, whereas the other cell has nearly the entire volume of cellular constituents. The smaller cell is called the first polar body, and the larger cell is referred to as the secondary oocyte.
During the second division of meiosis, a similar unequal cytokinesis takes place. Most of the cytoplasm is retained by the mature egg (ovum), and a second polar body receives little more than a haploid nucleus.
Thus, oogenic meiosis conserves the volume of oocyte cytoplasm in a single cell rather than splitting it equally among four progeny.
In a few species of animals, meiosis is severely modified such that the resulting gamete is diploid and need not be fertilized to develop. Such animals are said to be parthenogenetic (Greek, “virgin birth”).
In the fly Drosophila mangabeirai, one of the polar bodies acts as a sperm and “fertilizes” the oocyte after the second meiotic division. In other insects (such as Moraba virgo) and in the lizard Cnemidophorus uniparens, the oogonia double their chromosome number before meiosis, so that the halving of the chromosomes restores the diploid number.
The germ cells of the grasshopper Pycnoscelus surinamensis dispense with meiosis altogether, forming diploid ova by two mitotic divisions (Swanson et al. 1981). All of these species consist entirely of females.
In other species, haploid parthenogenesis is widely used not only as a means of reproduction, but also as a mechanism of sex determination. In the Hymenoptera (bees, wasps, and ants), unfertilized eggs develop into males, whereas fertilized eggs, being diploid, develop into females.
The haploid males are able to produce sperm by abandoning the first meiotic division, thereby forming two sperm cells through second meiosis.
Examples
Maturation of the oocyte in amphibians
The egg is responsible for initiating and directing development, and in some species (as seen above), fertilization is not even necessary. The accumulated material in the oocyte cytoplasm includes energy sources and energy-producing organelles (the yolk and mitochondria); the enzymes and precursors for DNA, RNA, and protein syntheses; stored messenger RNAs; structural proteins; and morphogenetic regulatory factors that control early embryogenesis. A partial catalogue of the materials stored in the oocyte cytoplasm, while a partial list of stored mRNAs. Most of this accumulation takes place during meiotic prophase I, and this stage is often subdivided into two phases, previtellogenesis (Greek, “before yolk formation”) and vitellogenesis.
The eggs of fishes and amphibians are derived from an oogonial stem cell population that can generate a new cohort of oocytes each year. In the frog Rana pipiens, oogenesis takes 3 years. During the first 2 years, the oocyte increases its size very gradually. During the third year, however, the rapid accumulation of yolk in the oocyte causes the egg to swell to its characteristically large size. Eggs mature in yearly batches, with the first cohort maturing shortly after metamorphosis; the next group matures a year later.
Vitellogenesis
Vitellogenesis occurs when the oocyte reaches the diplotene stage of meiotic prophase. Yolk is not a single substance, but a mixture of materials used for embryonic nutrition.
The major yolk component in frog eggs is a 470-kDa protein called vitellogenin. It is not made in the frog oocyte (as are the major yolk proteins of organisms such as annelids and crayfishes), but is synthesized in the liver and carried by the bloodstream to the ovary (Flickinger and Rounds 1956).
This large protein passes between the follicle cells of the ovary, and is incorporated into the oocyte by micropinocytosis, the pinching off of membrane-bounded vesicles at the bases of microvilli (Dumont 1978).
In the mature oocyte, vitellogenin is split into two smaller proteins: the heavily phosphorylated phosvitin and the lipoprotein lipovitellin.
These two proteins are packaged together into membrane-bounded yolk platelets. Glycogen granules and lipochondrial inclusions store the carbohydrate and lipid components of the yolk, respectively.
Most eggs are highly asymmetrical, and it is during oogenesis that the animal-vegetal axis of the egg is specified.
Danilchik and Gerhart (1987) have shown that although the concentration of yolk increases nearly tenfold as one moves from the animal to the vegetal poles of the mature Xenopus egg, vitellogenin uptake is uniform around the surface of the oocyte.
What varies is its movement within the oocyte, and this depends on where the yolk proteins enter. When yolk platelets are formed in the future animal hemisphere, they move inward toward the center of the cell.
Vegetal yolk platelets, however, do not actively move, but remain at the periphery of the cell for long periods of time, enlarging as they stay there. They are slowly displaced from the cortex as new yolk platelets come in from the surface.
As a result of this differential intracellular transport, the amount of yolk steadily increases in the vegetal hemisphere, until the vegetal half of a mature Xenopus oocyte contains nearly 75% of the yolk. The mechanism of this translocation remains unknown.
As the yolk is being deposited, the organelles also become arranged asymmetrically.
The cortical granules begin to form from the Golgi apparatus; they are originally scattered randomly through the oocyte cytoplasm, but later migrate to the periphery of the cell.
The mitochondria replicate at this time, dividing to form millions of mitochondria that will be apportioned to the different blastomeres during cleavage. (In Xenopus, new mitochondria will not be formed until after gastrulation is initiated.)
As vitellogenesis nears an end, the oocyte cytoplasm becomes stratified.
The cortical granules, mitochondria, and pigment granules are found at the periphery of the cell, within the actin-rich oocyte cortex.
Within the inner cytoplasm, distinct gradients emerge. While the yolk platelets become more heavily concentrated at the vegetal pole of the oocyte, the glycogen granules, ribosomes, lipid vesicles, and endoplasmic reticulum are found toward the animal pole.
Even specific mRNAs stored in the cytoplasm become localized to certain regions of the oocyte.
While the precise mechanisms for establishing these gradients remain unknown, studies using inhibitors have shown that the cytoskeleton is critically important in localizing specific RNAs and morphogenetic factors.
There seem to be two pathways for gettting mRNAs into the vegetal cortex (Forristall et al. 1995; Kloc and Etkin 1995, Kloc et al. 1998). The first pathway moves messages such as those encoding the Vg1 protein, which are initially present throughout the oocyte, into the vegetal cortex in a two-step process (Yisraeli et al. 1990).
In the first phase, microtubules are needed to bring Vg1 mRNA into the vegetal hemisphere. In the second phase, microfilaments are responsible for anchoring the Vg1 message to the cortex.
The portion of the Vg1 mRNA that binds to these cytoskeletal elements resides in its 3´ untranslated region. When a specific 340-base sequence from the Vg1 3´ UTR is placed onto a β-globin message, that β-globin mRNA is similarly localized to the vegetal cortex (Mowry and Melton 1992).
Other mRNAs, including germ plasm mRNAS such as Xlsirt and Xcat2, leave the germinal vesicle and join the mitochondrial “cloud” located at the vegetal pole of the nucleus.
These messages are compartmentalized into clusters associated with the germ plasm and transported to the vegetal cortex in a manner that appears to be independent of the cytoskeleton (Kloc et al. 1996). This mechanism is known as the Metro (message transport organizer) pathway.