CRISPR - Cas
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)- Cas (CRISPR-associated) nucleases system.
Introduction
Genome editing is the modification of genomic DNA at a specific target site in a wide variety of cell types and organisms, including insertion, deletion and replacement of DNA, resulting in inactivation of target genes, acquisition of novel genetic traits and correction of pathogenic gene mutations. In recent years, with the rapid development of life sciences, genome editing technology has become the most efficient method to study gene function, explore the pathogenesis of hereditary diseases, develop novel targets for gene therapy, breed crop varieties, and so on.
At present, there are three mainstream genome editing tools in the world, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and the RNA-guided CRISPR (clustered regularly interspaced short palindromic repeats)-Cas (CRISPR-associated) nucleases systems.
Due to the advantages of simple design, low cost, high efficiency, good repeatability and short-cycle, CRISPR-Cas systems have become the most widely used genome editing technology in molecular biology laboratories all around the world.
Overview
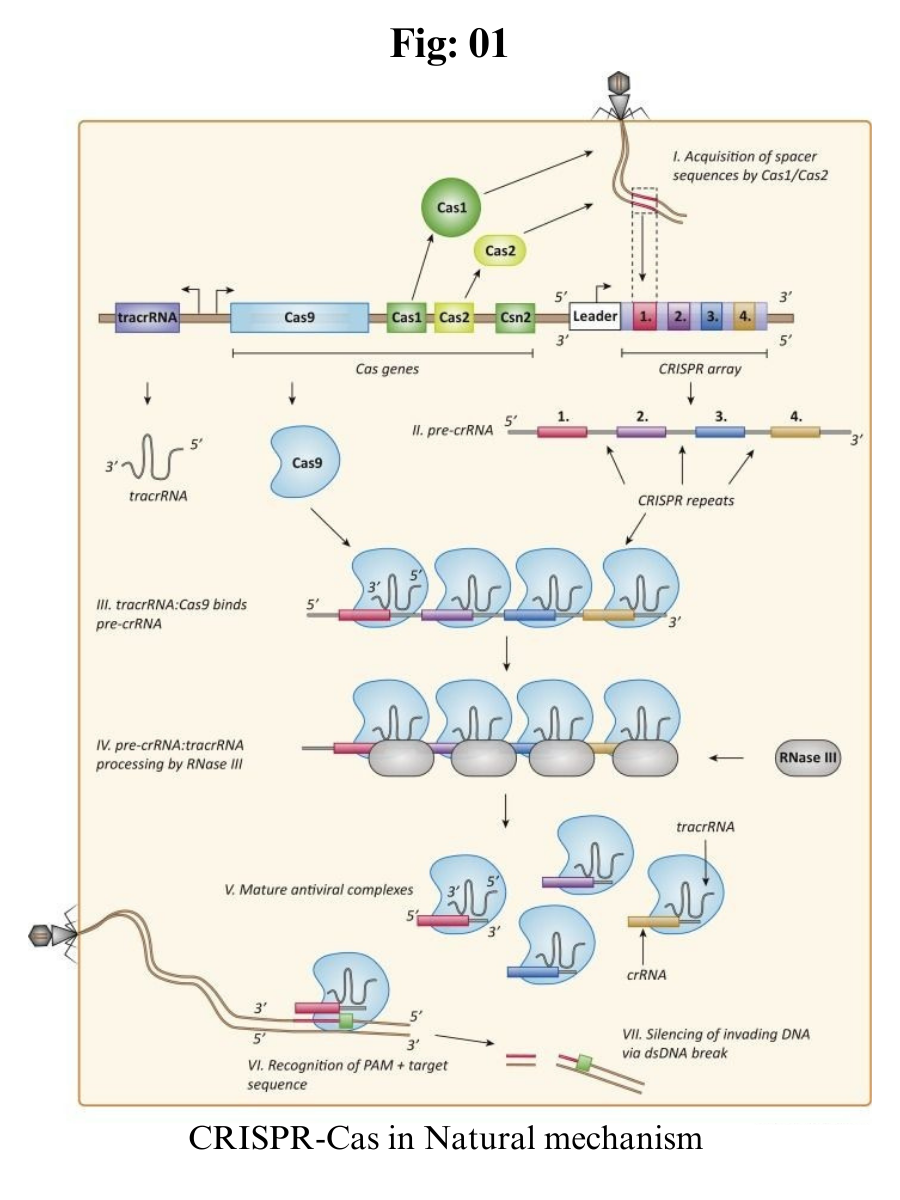
CRISPR-Cas is an adaptive immune system existing in most bacteria and archaea, preventing them from being infected by phages, viruses and other foreign genetic elements.
It is composed of CRISPR repeat-spacer arrays, which can be further transcribed into CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA), and a set of CRISPR-associated (cas) genes which encode Cas proteins with endonuclease activity.
When the prokaryotes are invaded by foreign genetic elements, the foreign DNA can be cut into short fragments by Cas proteins, then the DNA fragments will be integrated into the CRISPR array as new spacers.
Once the same invader invades again, crRNA will quickly recognize and pair with the foreign DNA, which guides Cas protein to cleave target sequences of foreign DNA, thereby protecting the host.
CRISPR Components
CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats of genetic information that some bacterial species use as part of an antiviral system. A group of scientists, including our co-founder Dr. Emmanuelle Charpentier, discovered how to use this system as a gene-editing tool.
Cas9: a CRISPR-associated (Cas) endonuclease, or enzyme, that acts as “molecular scissors” to cut DNA at a location specified by a guide RNA
Deoxyribonucleic acid (DNA): the molecule that most organisms use to store genetic information, which contains the “instructions for life”
Ribonucleic acid (RNA): a molecule related to DNA that living things use for a number of purposes, including transporting and reading the DNA “instructions”
Guide RNA (gRNA): a type of RNA molecule that binds to Cas9 and specifies, based on the sequence of the gRNA, the location at which Cas9 will cut DNA
PAM: An NGG PAM at the 3′ end of the target DNA site is essential for the recognition and cleavage of the target gene by Cas9 protein. Besides classical NGG PAM sites, other PAM sites such as NGA and NAG also exist, but their efficiency of genome editing is not high. However, such PAM sites only exist in about one-sixteenth of the human genome, thereby largely restricting the targetable genomic loci. For this purpose, several Cas9 variants have been developed to expand PAM compatibility.
Types of CRISPR-Cas
CRISPR-Cas systems can be classified into 2 classes (Class 1 and Class 2), 6 types (I to VI) and several subtypes, with multi-Cas protein effector complexes in Class 1 systems (Type I, III, and IV) and a single effector protein in Class 2 systems (Type II, V, and VI).
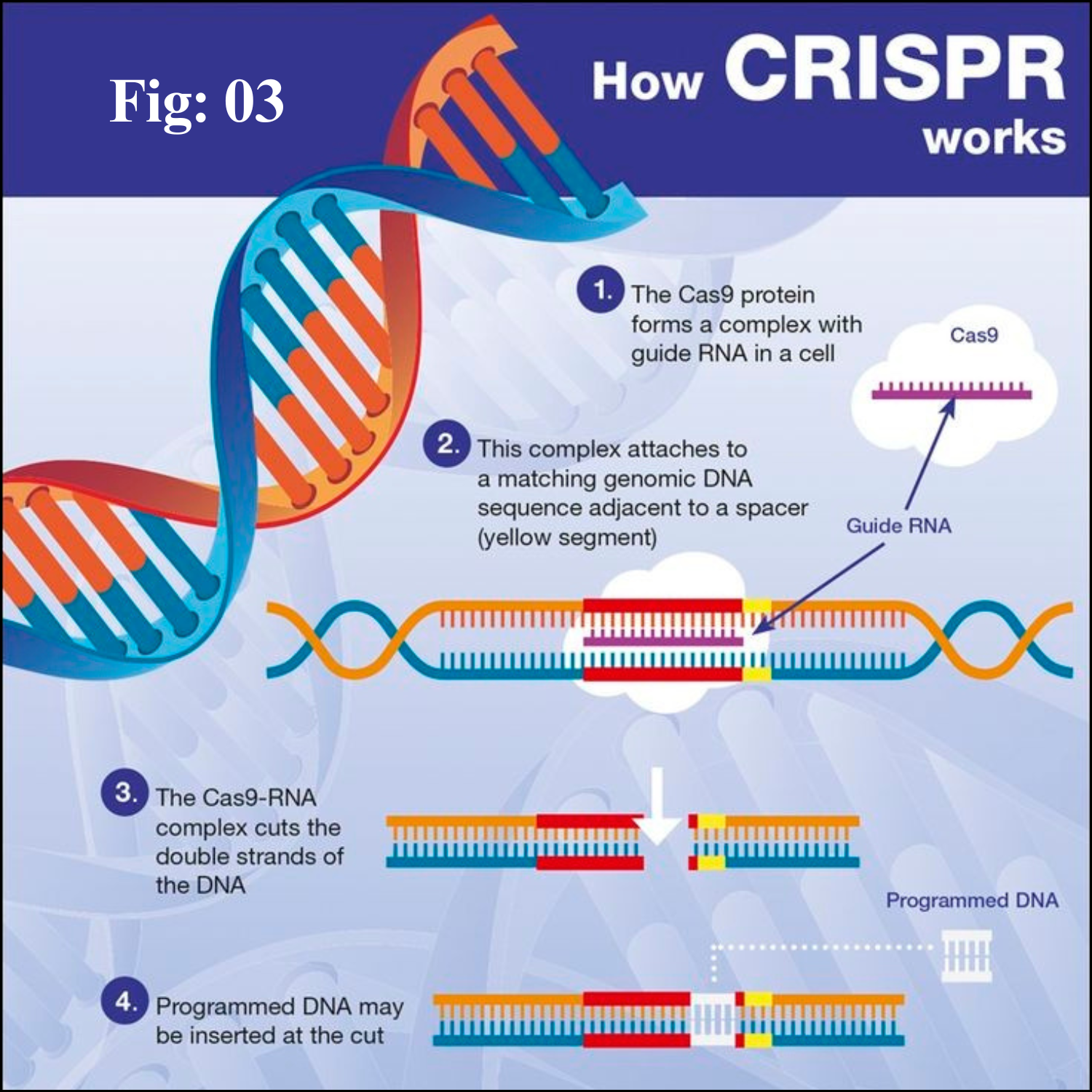
The Type II CRISPR-Cas9 system derived from Streptococcus pyogenes (SpCas9) is one of the best characterized and most commonly used categories in numerous CRISPR-Cas systems. The main components of the CRISPR-Cas9 system are RNA-guided Cas9 endonuclease and a single-guide RNA (sgRNA).
The Cas9 protein possesses two nuclease domains, named HNH and RuvC, and each cleaves one strand of the target double-stranded DNA. A single-guide RNA (sgRNA) is a simplified combination of crRNA and tracrRNA.
The Cas9 nuclease and sgRNA form a Cas9 ribonucleoprotein (RNP), which can bind and cleave the specific DNA target. Furthermore, a protospacer adjacent motif (PAM) sequence is required for Cas9 protein binding to the target DNA.
Process
During genome editing process, sgRNA recruits Cas9 endonuclease to a specific site in the genome to generate a double-stranded break (DSB), which can be repaired by two endogenous self-repair mechanisms, the error-prone non-homologous end joining (NHEJ) pathway or the homology-directed repair (HDR) pathway.
Under most conditions, NHEJ is more efficient than HDR, for it is active in about 90% of the cell cycle and not dependent on nearby homology donors. NHEJ can introduce random insertions or deletions (indels) into the cleavage sites, leading to the generation of frameshift mutations or premature stop codons within the open reading frame (ORF) of the target genes, finally inactivating the target genes.
Alternatively, HDR can introduce precise genomic modifications at the target site by using a homologous DNA repair template. Furthermore, large fragment deletions and simultaneous knockout of multiple genes could be achieved by using multiple sgRNAs targeting one single gene or more.
Applications of CRISPR-Cas systems in gene therapy
Gene therapy refers to the introduction of foreign genes into target cells to treat specific diseases caused by mutated or defective genes.
Target cells of gene therapy are mainly divided into two categories: somatic cells and germ line cells. However, since germ line gene therapy is complicated in technique as well as involves ethical and security issues, today gene therapy is limited to somatic cell gene therapy.
Traditional gene therapy is usually carried out by homologous recombination or lentiviral delivery. Nevertheless, the efficiency of homologous recombination is low, and lentiviral vectors are randomly inserted into the recipient genome, which may bring potential security risks to clinical applications.
Currently, with the rapid development of CRISPR-Cas systems, they have been widely applied in gene therapy for treating various human diseases, monogenic diseases, infectious diseases, cancer, etc.
Furthermore, some CRISPR-mediated genome-editing therapies have already reached the stage of clinical testing. Table 4 briefly summarizes the ongoing clinical trials of gene therapy using genome-editing technology, including ZFN, TALEN and CRISPR-Cas systems.
β-Thalassaemia
β-Thalassaemia, a hereditary hemolytic anemia disease, is one of the most common and health-threatening monogenic diseases in the world. It is characterized by mutations in the β-globin (HBB) gene, leading to severe anemia caused by decreased hemoglobin (Hb) level.
For the moment, the only way to cure β-thalassemia is hematopoietic stem cell transplantation (HSCT). Yet, high cost of treatment and shortage of donors limit its clinical application. Other therapy, for example, blood transfusion, can only sustain the life of patients but can’t cure the disease.
To better treat β-thalassemia, researchers have turned their attention to gene therapy. A major technical idea is to repair the defective β-globin gene of iPSCs from patients with β-thalassemia by CRISPR-Cas9 technology, then red blood cells can be produced normally and the disease could be cured.
Besides, reactivating fetal hemoglobin (HbF) expression has also been proposed to be an effective method to treat β-thalassemia through knockout of BCL11A gene, which suppresses the expression of fetal hemoglobin.
Sickle Cell Disease
Additionally, CRISPR-Cas systems have also been used for the treatment of other hematologic diseases, such as sickle cell disease (SCD) and hemophilia B (HB).
SCD is a monogenic disease caused by a single-nucleotide mutation in human β-globin gene, leading to a substitution of glutamic acid by valine and the production of an abnormal version of β-globin, which is known as hemoglobin S (HbS).
CRISPR-Cas9 system has been used to treat SCD by repairing the β-globin gene mutation or reactivating HbF expression.
HB is an X-linked hereditary bleeding disorder caused by deficiency of coagulation factor IX, and the most common treatment for hemophilia B is supplement blood coagulation factor.
Huai et al. injected naked Cas9-sgRNA plasmid and donor DNA into the adult mice of F9 mutation HB mouse model for gene correction.
Meanwhile, Cas9/sgRNA were also microinjected into germline cells of this HB mouse model for gene correction. Both in vivo and ex vivo experiments were sufficient to resolve the coagulation deficiency.
Guan et al. corrected the F9 Y371D mutation in HB mice using CRISPR-Cas9 mediated in situ genome editing, which greatly improved the hemostatic efficiency and increased the survival of HB mice.
HIV (Human immunodeficiency virus)
Human immunodeficiency virus (HIV), a kind of retrovirus, mainly attacks the human immune system, especially the CD4+ T lymphocytes. When human cells are invaded by HIV, the viral sequences can be integrated into the host genome, blocking cellular and humoral immunity while causing acquired immunodeficiency syndrome (AIDS).
There is still no known cure for AIDS but it could be treated. Although antiretroviral therapy can inhibit HIV-1 replication, the viral sequences still exist in the host genome, and they could be reactivated at any time.
CRISPR-Cas9 system can target long terminal repeat (LTR) and destruct HIV-1 proviruses, thus it is possible to completely eliminate HIV-1 from the genome of infected host cells. In addition, resistance to HIV-1 infection could be induced by knockout of the HIV co-receptor CCR5 gene in CD4+ T cells.
HPV (Human Papilloma Virus)
Cervical cancer is the second most common gynecologic malignant tumor. The incidence is increasing year by year and young people are especially prone to this disease. It was found that the occurrence of cervical cancer is closely related to HPV (human papillomavirus) infection.
HPV is a double-stranded cyclic DNA virus, E6 and E7 genes located in HPV16 early regions are carcinogenic genes. Researchers designed sgRNAs targeting E6 and E7 genes to block the expression of E6 and E7 protein, subsequently the expression of p53 and pRb was restored to normal, finally increasing tumor cells apoptosis and suppressing subcutaneous tumor growth in in vivo experiments. Moreover, HPV virus proliferation was blocked through cutting off E6/E7 genes, and the virus in the bodies could be eliminated.
Cancer
Cancer is the second leading cause of death worldwide after cardiovascular diseases, and it is also a medical problem that needs to be solved urgently. A variety of genetic or epigenetic mutations have been accumulated in the cancer genome, which can activate proto-oncogenes, inactivate tumor suppressors and produce drug resistance.
So far, CRISPR-Cas systems have been used to correct the oncogenic genome/epigenome mutations in tumor cells and animal models, resulting in inhibition of tumor cell growth and promotion of cell apoptosis, thereby inhibiting tumor growth.
Delivery methods
Nowadays, how to effectively deliver CRISPR-Cas components to specific cells, tissues and organs for precisely directed genome editing is still a major problem in gene therapy. Ideal delivery vectors should have the advantages of non-toxicity, well targeting property, high efficiency, low cost, and biodegradability.
At present, three main delivery methods have been employed in delivering CRISPR-Cas components, including physical, viral and non-viral methods.
Physical methods are the simplest way to deliver CRISPR-Cas components, including electroporation, microinjection and mechanical cell deformation. They are simple and efficient, which can also improve the expression of genes, and are widely applied in in vitro experiments.
In addition, viral vectors, such as adenovirus, adeno-associated virus (AAV) and lentivirus viral vectors, are being widely used for both in vitro/ex vivo and in vivo delivery due to their high delivery efficiency.
They are commonly used for gene delivery in gene therapy, and some of them have been approved for clinical use. However, the safety issue of viral vectors is still a major problem that needs to be solved in pre-clinical trials.
Therefore, researchers have turned their attention to non-viral vectors, for instance, liposomes, polymers and nanoparticles. Based on the advantages of safety, availability and cost-effectiveness, they are becoming a hotspot for the delivery of CRISPR-Cas components.
Ethical Issues
CRISPR-Cas mediated genome editing has attracted much attention since its advent in 2012. In theory, each gene can be edited by CRISPR-Cas systems, even genes in human germ cells. However, germline gene editing is forbidden in many countries including China, for it could have unintended consequences and bring ethical and safety concerns.
However, in March 2015, a Chinese scientist, Junjiu Huang, published a paper about gene editing in human tripronuclear zygotes in the journal Protein & Cell, which brings the ethical controversy of human embryo gene editing to a climax. Since then, genome editing has been challenged by ethics and morality, and legal regulation of genome editing has triggered a heated discussion all around the world.
Then, on Nov. 28, 2018, the day before the opening of the second international human genome editing summit, Jiankui He, a Chinese scientist from the Southern University of Science and Technology, announced that a pair of gene-edited babies, named Lulu and Nana, were born healthy in China this month. They are the world’s first gene-edited babies, whose CCR5 gene has been modified, making them naturally resistant to HIV infection after birth. The announcement has provoked shock, even outrage among scientists around the world, causing widespread controversy in the application of genome editing.
The society was shocked by this breaking news, for it involves genome editing in human embryos and propagating into future generations, triggering a chorus of criticism from the scientific community and bringing concerns about ethics and security in the use of genome editing.
Therefore, scientists call on the Chinese government to investigate the matter fully and establish strict regulations on human genome editing. Global supervisory system is also needed to ensure genome editing of human embryos moving ahead safely and ethically.
Conclusion
Since CRISPR-Cas mediated genome editing technologies have provided an accessible and adaptable means to alter, regulate, and visualize genomes, they are thought to be a major milestone for molecular biology in the 21st century.
So far, CRISPR-Cas systems have been broadly applied in gene function analysis, human gene therapy, targeted drug development, animal model construction and livestock breeding, which fully prove their great potential for further development.
However, there are still some limitations to overcome in the practical applications of CRISPR-Cas systems, and great efforts still need to be made to evaluate their long-term safety and effectiveness.