Ribosomes
Introduction:
Ribosomes are tiny, granular organelles found in both eukaryotic and prokaryotic cells. They are found inside the cytosol of the cell and play. an important role in protein synthesis by translating the genetic information conveyed by messenger RNA (mRNA) into functional proteins. Ribosomes are composed of two subunits, one larger and one smaller, each of which is made up of proteins and RNA molecules.
"A ribosome is a cellular structure that assembles proteins by linking together amino acids based on genetic instructions from messenger RNA (mRNA)."
Ribosomes were first observed by George Palade (1953) under the electron microscope. It is a kind of complex molecular machine present inside the living cells that produce proteins from amino acids during a process of protein synthesis also called translation. Ribosome translates genetic information stored in messenger RNA into proteins.
The process occur in three stages: initiation, elongation and termination. Within ribosomes, ribosomal RNA (rRNA) catalyzes the peptidyl transferase reaction, to form peptide bonds between amino acids, enabling them to form proteins. After protein is formed in the ribosome, they move to different areas of the cell for various cellular functions.
Ribosomes Location:
Ribosomes are present in the cytosol or attached to the endoplasmic reticulum in both plant and animal cells. They play an important role in translating DNA into proteins. While some ribosomes are permanently associated with the rough endoplasmic reticulum, their association depends on the specific proteins they help to produce. In animal or human cells, there can be as many as 10 million ribosomes. Multiple ribosomes can be linked to the same mRNA strand, a structure known as a Polysome.
Occurrence of Ribosomes:
Ribosomes are distributed universally all throughout the kingdom of animals and plants. They're also found in prokaryotes. The mammalian RBC is the only cell type devoid of ribosomes. For any given type, the density of ribosomes per unit area is rather constant. It is high in active m protein synthesis cells and low in cells where synthesis of protein is low.
Distribution of Ribosomes:
The ribosomes frequently occur freely in the cytoplasm in prokaryotic cells. The ribosomes occur freely in the cytoplasm in eukaryotic cells or remain attached to the outer surface of the endoplasmic reticulum (ER) membrane. They are called free ribosomes if they are not attached to the ER. Free ribosomes represent protein synthesis sites needed to maintain the cytoplasmic matrix's enzyme composition.
Number and Concentration of Ribosomes:
Ribosomes can be observed in all cells containing endoplasmic reticulum. Nearly 100 ribosomes per μ3 are found in rabbit reticulocytes, which corresponds to 1x105 particles per reticulocyte and approximately. 5% of the total cell mass, or approximately 20,000 to 30,000 cell mass. But, if unfavorable nutritional conditions slow the rate of protein synthesis, the number of ribosomes can drop significantly in protein synthesizing cells and bacteria.
Chemical Composition of Ribosomes:
RNA and proteins are the main constituents of ribosomes. The lipids are completely absent or traceable. E. Coli's ribosomes possess almost 60-65% of RNA and 35-40% of their weight protein. Ribosomal RNA differs from tRNA and other RNA classes of most cells in size and base content. In all ribosomes, two types of RNAs are found. They are an essential component and can't be easily removed.
Ribosome Structure:
The structure of the ribosome is described as follows
Ribosomes consist of both ribonucleic acid (RNA) and protein part. The RNA component is called ribosomal RNA (rRNA), and the protein component consists of various ribosomal proteins.
Ribosomes consists of two subunits – a small subunit and a large subunit and these subunits work together for the process of protein synthesis.
The small subunit reads the genetic information and binds to mRNA. The large subunit catalyzes peptide bond formation and binds to the aminoacylated tRNAs.
Ribosome has specific binding sites for different molecules involved in protein synthesis. These include: A (aminoacyl) site: The site where aminoacylated tRNA molecules are accepted. P (peptidyl) site: contains the tRNA which carries the growing peptide chain. E (exit) site: the site where deacylated tRNA molecules remains before leaving the ribosome.
The ribosomes when attached to the endoplasmic reticulum, it is called the rough endoplasmic reticulum.
Bound and free ribosomes are similar in structure, and they are involved in protein synthesis.
Characteristics of Ribosomes:
Ribosomes is a cellular structures that take part in the protein synthesis in all living organisms. The characteristics of ribosomes is as follows
Ribosomes are found in both prokaryotic or eukaryotic organisms.
Ribosomes are composed of ribonucleic acid (RNA) and proteins. The RNA component is called ribosomal RNA (rRNA).
Ribosomes consist of two subunits, a small subunit and a large subunit. Both work together during protein synthesis.
Ribosomes have specific binding sites for molecules carrying out protein synthesis.
Ribosomes are found in two regions of the cell: scattered throughout the cytoplasm and attached to the endoplasmic reticulum in some cases, and form the rough endoplasmic reticulum.
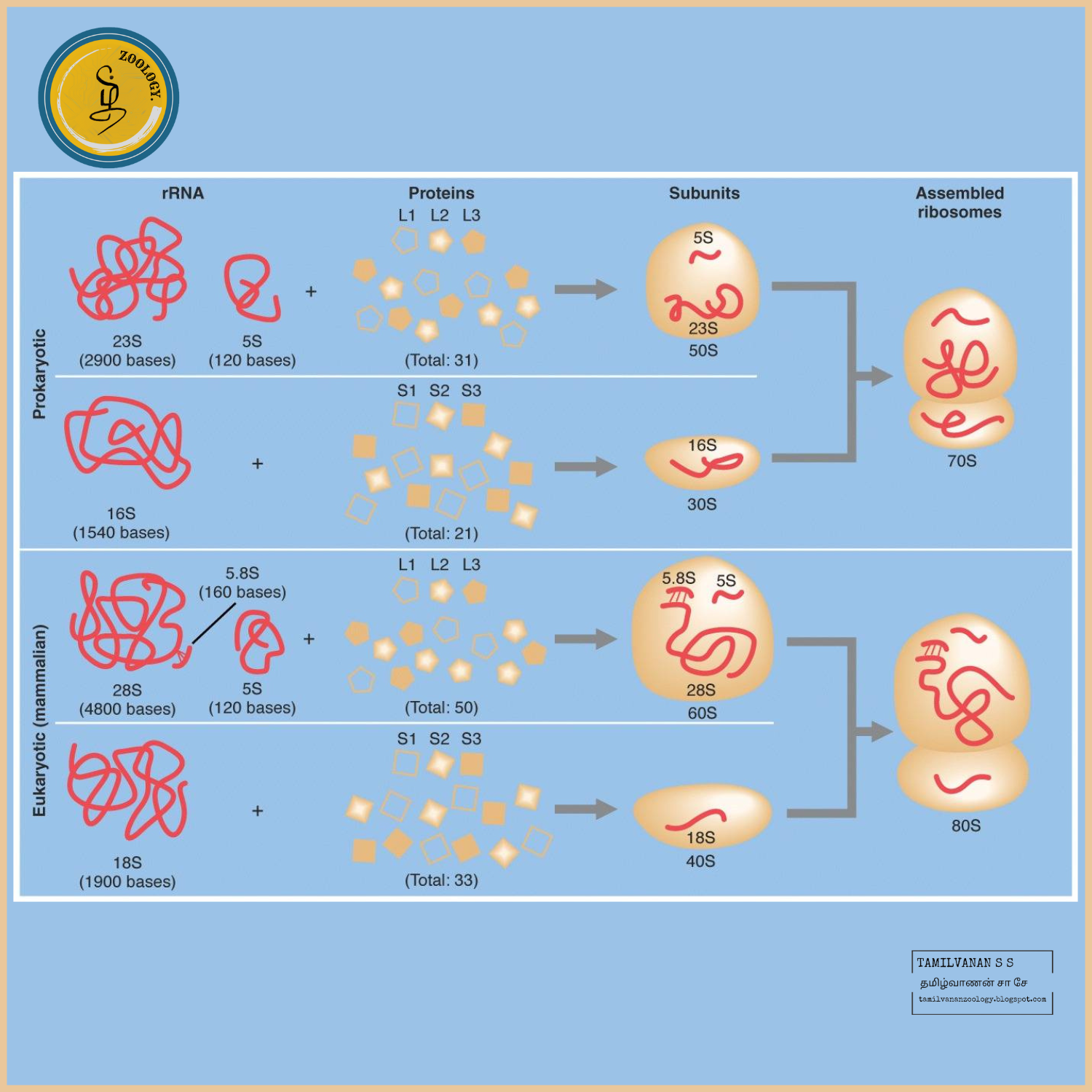
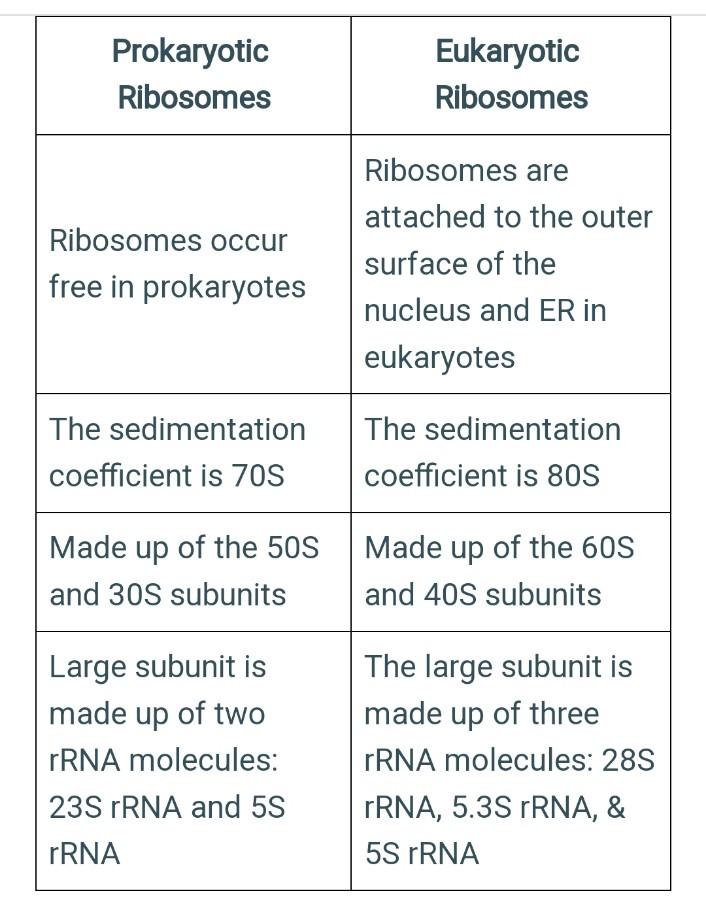
Prokaryotes possesses 70S ribosomes, consisting of a small subunit (30S) and a large subunit (50S). Eukaryotes possess 80S ribosomes, with a small subunit (40S) and a large subunit (60S).
They read the genetic code carried by messenger RNA (mRNA) and use it to assemble amino acids into a specific sequence, ultimately forming proteins.
Prokaryotic Ribosomes:
As compared to eukaryotic ribosomes, the prokaryotic ribosomes are smaller. This is attributed to the association of eukaryotic cell ribosomes with the cytoplasmic or endoplasmic reticulum.
Prokaryotic ribosomes are called 70S ribosomes and have physical dimensions of approximately 14 to 15 nm by 20 nm, with a molecular weight of roughly 2.7 million, and are made of 50S and 30S subunits.
The S stands for Svedberg unit in the measurement of ribosome sedimentation coefficient unit. This is a measure of the velocity of sedimentation in a centrifuge; the faster a particle travels when centrifuged, the higher its Svedberg value or the coefficient of sedimentation.
The coefficient of sedimentation is a function of the molecular weight, volume, and shape of a particle. Normally, heavier and more compact particles have greater numbers of Svedberg or faster sediments.
There are smaller bacterial ribosomes than eukaryotic ribosomes. A prokaryotic cell usually only has a few thousand ribosomes, while there are several million in a metabolically active eukaryotic cell, such as a human liver cell.
Proteins that work in the cytoplasm are produced by free ribosomes that are suspended there, while proteins that are bound within membranes or intended for export from the cell are assembled by ribosomes that are bound to rough ER.
The large subunit, called the 50S, is spherical in the prokaryotic cell with a prominent "stalk" and a "central protuberance." It contains the center of peptidyltransferase which catalyzes the formation of peptide bonds between the incoming amino acid and the growing chain of peptides.
The 50S particle is thick and homogenous. The thin and flexible small subunit of ribosomes in prokaryotes is called the 30S. The 30S houses the decoding center for mRNA and is divided into three domains i.e. head, body, and platform.
Each one of these domains includes one of the 16S rRNA's main secondary structure domains, namely the 3' major, 5' and central domains. In 16S rRNA, 3' minor domain forms an extended helix running down the 30S subunit surface's long axis, which interacts with the 50S subunit.
All four 30S particle domains join in a relatively narrow region of the neck. The two "active sites'' face each other around the interface of the subunit and are physiologically linked by the molecule's two ends.
Eukaryotic Ribosomes:
The eukaryotic ribosome (i.e., one not found in mitochondria and chloroplasts) is larger than the prokaryotic 70S ribosome. It is a dimer of the 60S and the 40S subunit, about 22 nm in diameter, and has the sedimentation coefficient of 80S and a molecular weight of 4 million.
Eukaryotic ribosomes can be either associated with the endoplasmic reticulum or free in the cytoplasmic matrix. When bound to the endoplasmic reticulum to form rough ER, they are attached through their 60S subunits.
Both free and ER-bound ribosomes synthesize proteins. Proteins made on the ribosomes of the RER are often secreted or are inserted into the ER membrane as integral membrane proteins.
Free ribosomes are the sites of synthesis for non secretory and non membrane proteins. Some proteins synthesized by free ribosomes are inserted into organelles such as the nucleus, mitochondrion, and chloroplast. They also assist the transport of proteins into eukaryotic organelles such as mitochondria
Types of rRNA
In Prokaryotes
In prokaryotes, the 16S ribosomal RNA is housed in a compact 30S ribosomal subunit. Two rRNA species are present in the large 50S ribosomal subunit (the 23S and 5S ribosomal RNAs). Therefore, it may be concluded that a single rRNA gene in archaea and bacteria codes for the three rRNA types: 16S, 23S, and 5S.
The bacterial 23S, 16S, and 5S rRNA genes are commonly arranged as a co-transcribed operon.
In Eukaryotes
The eukaryotic ribosome is composed of the 40S and a 60S subunit. Eukaryotes, in contrast, usually have several variants of the rRNA genes arranged in repetitive sequences. About 300-400 repeats are found in five clusters on human chromosomes: 13 (RNR1), 14 (RNR2), 15 (RNR3), 21 (RNR4) and 22 (RNR5).
Furthermore, chloroplasts and mitochondria in eukaryotic cells both contain rRNA. Ribosomes can be found in the cytoplasm as free-floating complexes or connected to the endoplasmic reticulum.
Functions of rRNA:
Protein synthesis is the primary function of rRNA. The A, P, and E sites are created within the ribosome by the unusual three-dimensional structure of rRNA, which has internal helices and loops. By attaching to messenger RNA and transfer RNA, these molecules assure that the codon sequence of the mRNA is appropriately translated into the amino acid sequence of proteins.
The A site anchors an entering tRNA that has been charged with an amino acid, while the P site is for binding a developing polypeptide. The tRNA temporarily attaches to the E site following the creation of a peptide bond before exiting the ribosome.
In addition, some ribosomal proteins can bind to rRNA at specific residues, which have been identified after detailed investigation for both the RNA and protein.
Antibiotics like streptomycin and tetracycline have recently been identified as having binding sites on bacterial rRNA. For example, a mutation in the 16S rRNA sequence is the tolerance of Euglena and Escherichia coli to streptomycin.
The 30S rRNA appears to be the source of tetracycline resistance. Similar findings were discovered for Streptomyces to Spectinomycin resistance.
Preribosomal RNA, one of rRNA’s predecessors, has been linked to the production of microRNA, which mediates inflammation and heart illness concerning mechanical stress. This finding adds a new dimension to the role of rRNA.
Ribosomes Functions:
Ribosomes have two principal functions, which involve decoding the messages and the formation of peptide bonds.
Ribosomes participate in the creation of proteins, the DNA makes RNA by DNA transcription.
The mRNA is converted into proteins by the process of translation.
The mRNA is organized in the nucleus and is moved to the cytoplasm for the process of protein synthesis.
The ribosomal subunits in the cytoplasm are bound around mRNA polymers. The tRNA then integrates proteins.
The proteins organized in the cytoplasm are used in the actual cytoplasm, and the proteins synthesized by bound ribosomes are moved to external cells.
Ribosome Associated Diseases:
Disorders caused by the improper functioning of ribosomes are called ribosomopathies. Mutations that occur in some of the proteins made by ribosomes can cause disorders that are characterized by like bone marrow failure and anemia.
Many congenital syndromes are caused by defective ribosome biogenesis, which includes Diamond-Blackfan anemia (DBA), X-linked dyskeratosis congenita (DKC), cartilage hair hypoplasia (CHH), and Treacher Collins syndrome (TCS).
Diamond-Blackfan anemia (DBA)
It is a rare blood disorder that affects the bone marrow. The bone marrow can make fresh blood cells, including red blood cells, white blood cells, and platelets. In DBA, the bone marrow can’t make sufficient RBC to address the body’s issues. DBA is described as a deficiency of RBC that causes anaemia.
DBA causes abnormal pre-rRNA maturation and shows mutations in one of several ribosomal protein genes that encode structural components of the ribosome.
Summary:
All prokaryotes have 70S ribosomes whereas eukaryotes in their cytosol contain larger 80S ribosomes.
The 70S ribosome consists of subunits 50S and 30S. In catalyzing two biological processes, ribosomes play a key role in the transfer of peptidyl and hydrolysis of peptidyl.
The eukaryotic ribosome (i.e., one not found in mitochondria and chloroplasts) is larger than the prokaryotic 70S ribosome.
At any given moment, many rRNA molecules dangle from the chromosome at the sites of these clusters of genes that encode rRNA.
The ribosome reading of each codon results in the incorporation of one amino acid into a progressively longer protein chain. The transfer of RNA (tRNA) molecules, which are the adapter molecules in the translation mechanism, brings the amino acids to the ribosome.