Introduction
While the reductive divisions of meiosis are conserved in every eukaryotic kingdom of life, the regulation of meiosis in mammals differs dramatically between males and females. The differences between oogenesis, the production of eggs, and spermatogenesis.
Spermatogenesis
Spermatogenesis is the production of sperm from the primordial germ cells. Once the vertebrate PGCs arrive at the genital ridge of a male embryo, they become incorporated into the sex cords. They remain there until maturity, at which time the sex cords hollow out to form the seminiferous tubules, and the epithelium of the tubules differentiates into the Sertoli cells. The initiation of spermatogenesis during puberty is probably regulated by the synthesis of BMP8B by the spermatogenic germ cells, the spermatogonia. When BMP8B reaches a critical concentration, the germ cells begin to differentiate. The differentiating cells produce high levels of BMP8B, which can then further stimulate their differentiation. Mice lacking BMP8B do not initiate spermatogenesis at puberty (Zhao et al. 1996).
Process
The spermatogenic germ cells are bound to the Sertoli cells by N-cadherin molecules on both cell surfaces and by galactosyltransferase molecules on the spermatogenic cells that bind a carbohydrate receptor on the Sertoli cells (Newton et al. 1993; Pratt et al. 1993). The Sertoli cells nourish and protect the developing sperm cells, and spermatogenesis—the developmental pathway from germ cell to mature sperm—occurs in the recesses of the Sertoli cells. The processes by which the PGCs generate sperm have been studied in detail in several organisms, but we will focus here on spermatogenesis in mammals.
After reaching the gonad, the PGCs divide to form type A1 spermatogonia. These cells are smaller than the PGCs and are characterized by an ovoid nucleus that contains chromatin associated with the nuclear membrane. The A1 spermatogonia are found adjacent to the outer basement membrane of the sex cords. They are stem cells, and at maturity, they are thought to divide so as to make another type A1 spermatogonium as well as a second, paler type of cell, the type A2 spermatogonium. Thus, each type A1 spermatogonium is a stem cell capable of regenerating itself as well as producing a new cell type. The A2 spermatogonia divide to produce the A3 spermatogonia, which then beget the type A4 spermatogonia. It is possible that each of the type A spermatogonia are stem cells, capable of self-renewal. The A4 spermatogonium has three options: it can form another A4 spermatogonium (self-renewal); it can undergo cell death (apoptosis); or it can differentiate into the first committed stem cell type, the intermediate spermatogonium. Intermediate spermatogonia are committed to becoming spermatozoa, and they divide mitotically once to form the type B spermatogonia. These cells are the precursors of the spermatocytes and are the last cells of the line that undergo mitosis. They divide once to generate the primary spermatocytes—the cells that enter meiosis. It is not known what causes the spermatogonia to take the path toward differentiation rather than self-renewal; nor is it known what stimulates the cells to enter meiotic rather than mitotic division (Dym 1994).
We find that during the spermatogonial divisions, cytokinesis is not complete. Rather, the cells form a syncytium whereby each cell communicates with the others via cytoplasmic bridges about 1 μm in diameter (Dym and Fawcett 1971). The successive divisions produce clones of interconnected cells, and because ions and molecules readily pass through these intercellular bridges, each cohort matures synchronously. During this time, the spermatocyte nucleus often transcribes genes whose products will be used later to form the axoneme and acrosome.
Each primary spermatocyte undergoes the first meiotic division to yield a pair of secondary spermatocytes, which complete the second division of meiosis. The haploid cells thus formed are called spermatids, and they are still connected to one another through their cytoplasmic bridges. The spermatids that are connected in this manner have haploid nuclei, but are functionally diploid, since a gene product made in one cell can readily diffuse into the cytoplasm of its neighbors (Braun et al. 1989). During the divisions from type A1 spermatogonium to spermatid, the cells move farther and farther away from the basement membrane of the seminiferous tubule and closer to its lumen. Thus, each type of cell can be found in a particular layer of the tubule. The spermatids are located at the border of the lumen, and here they lose their cytoplasmic connections and differentiate into sperm cells. In humans, the progression from spermatogonial stem cell to mature sperm takes 65 days (Dym 1994).
Spermiogenesis
The mammalian haploid spermatid is a round, unflagellated cell that looks nothing like the mature vertebrate sperm. The next step in sperm maturation, then, is spermiogenesis (or spermateliosis), the differentiation of the sperm cell. For fertilization to occur, the sperm has to meet and bind with the egg, and spermiogenesis prepares the sperm for these functions of motility and interaction. The first steps involve the construction of the acrosomal vesicle from the Golgi apparatus. The acrosome forms a cap that covers the sperm nucleus. As the acrosomal cap is formed, the nucleus rotates so that the cap will be facing the basal membrane of the seminiferous tubule. This rotation is necessary because the flagellum is beginning to form from the centriole on the other side of the nucleus, and this flagellum will extend into the lumen. During the last stage of spermiogenesis, the nucleus flattens and condenses, the remaining cytoplasm (the “cytoplasmic droplet”) is jettisoned, and the mitochondria form a ring around the base of the flagellum.
One of the major changes in the nucleus is the replacement of the histones by protamines. Transcription of the gene for protamine is seen in the early haploid cells (spermatids), although translation is delayed for several days (Peschon et al. 1987). Protamines are relatively small proteins that are over 60% arginine. During spermiogenesis, the nucleosomes dissociate, and the histones of the haploid nucleus are eventually replaced by protamines. This causes the complete shutdown of transcription in the nucleus and facilitates its assuming an almost crystalline structure. The resulting sperm then enter the lumen of the tubule.
In the mouse, the entire development process from stem cell to spermatozoon takes 34.5 days. The spermatogonial stages last 8 days, meiosis lasts 13 days, and spermiogenesis takes up another 13.5 days. In humans, spermatic development takes nearly twice as long to complete. Because the type A1 spermatogonia are stem cells, spermatogenesis can occur continuously. Each day, some 100 million sperm are made in each human testicle, and each ejaculation releases 200 million sperm. Unused sperm are either resorbed or passed out of the body in urine. During his lifetime, a human male can produce 1012 to 1013 sperm (Reijo et al. 1995).
Kind of Abnormalities in Sperm
The abnormalities found in the sperm include:
Aspermia
This is a condition where a man experiences dry orgasms or orgasms without releasing any semen. This may be the result of retrograde ejaculation, genetic disorders like cystic fibrosis or Klinefelter syndrome, hormonal imbalances or congenital abnormalities. This condition may affect male fertility.
Hypospermia
In such conditions, the total ejaculate is less than 1.5 millilitres. Retrograde ejaculation is the most common cause of this condition. However, it may also be caused by genetic disorders, hormonal imbalances or congenital abnormalities.
Azoospermia
This is a condition wherein a man releases semen that contains no sperm during an orgasm. It is a severe type of male infertility. It may be caused by genetic disorders, hormonal imbalances, congenital abnormalities, untreated STDs and because of post-testicular cancer treatment.
Oligozoospermia
This is a condition wherein the man may have a low sperm count as well as problems with sperm shape and sperm movement. This may be caused by a varicocele vein, hormonal imbalances, undescended testicles, infections of the reproductive tract, environmental conditions and lifestyle choices. In some cases, making a few lifestyle changes may help improve the condition.
Asthenozoospermia
This is a condition where a large percentage of sperm moves abnormally i.e., it doesn’t move in a straight line or does not move at all. This condition may be accompanied by a low sperm count. Some of the causes of this include exposure to toxins, nutritional problems, excessive alcohol consumption, infections and side effects of certain medications.
Teratozoospermia
In his condition, the majority of the sperm in the semen are shaped abnormally. The sperms may have more than a single head or tail or an oddly shaped head. This keeps them from moving normally and affects their ability to fertilise an egg.
Oligoasthenoteratozoospermia (OAT)
In such cases the shape, size and movement of the sperm are abnormal. The sperm count may also be lower than normal. This is the most common cause of male infertility.
Necrozoospermia
In such cases all the sperm in the semen is dead. It is a rare cause of infertility.
Leukocytospermia
This is a condition where there is a high amount of white blood cells present in the semen. It is more of a semen abnormality than a sperm abnormality.
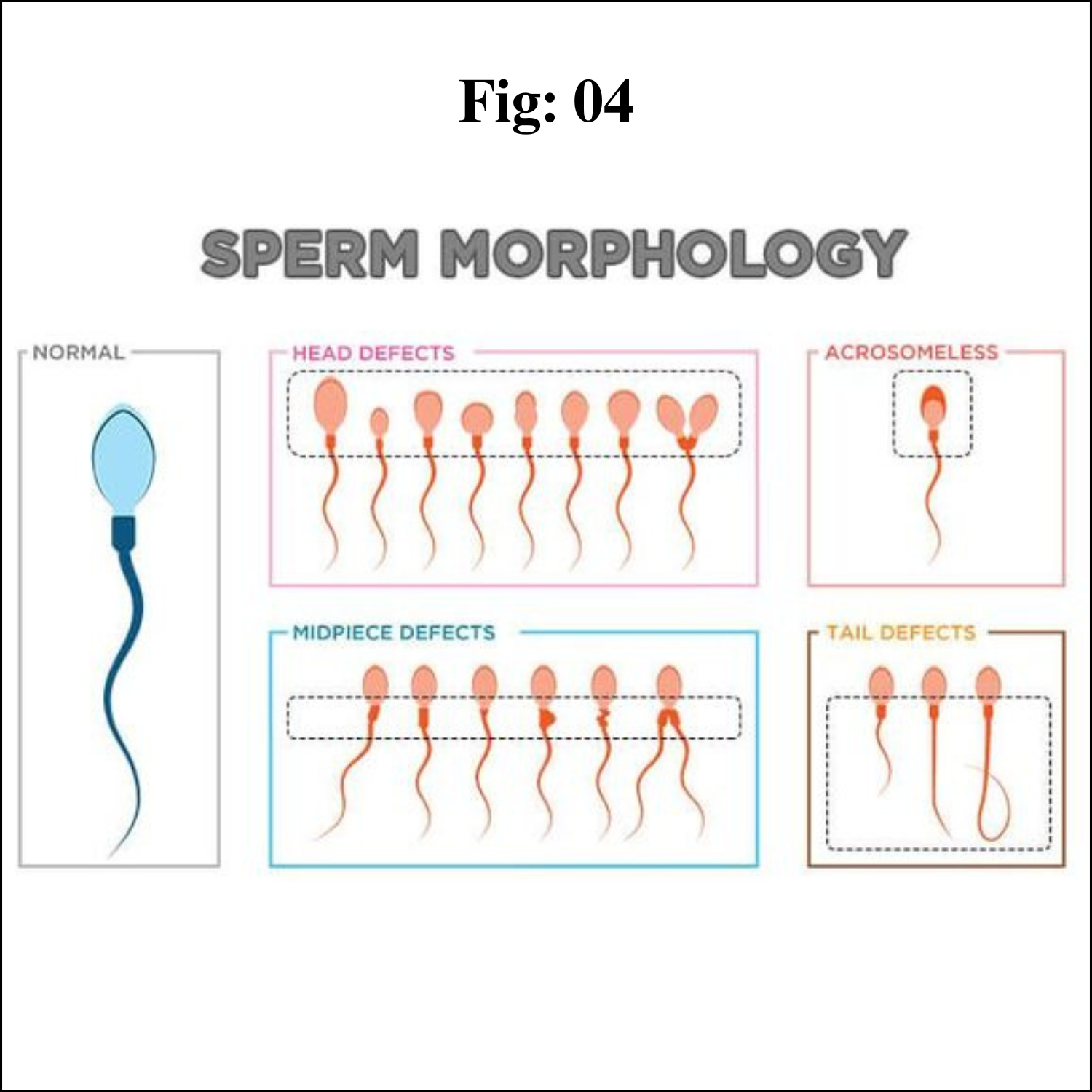
Abnormal Sperm Morphology
Teratozoospermia, teratospermia or abnormal sperm morphology is a semen alteration in which a large percentage of sperm have an abnormal shape.
Abnormal Sperm Morphology and Male Infertility
In order for a man to be fertile, only 4 to 14% of normal sperm is needed. Just having abnormal sperm won’t have any impact on fertility; there are other factors. Also, they are sperm numbers, sperm concentration, semen volume, the percentage of sperm that are alive (viability) and the ability to move (motility). An abnormally shaped sperm doesn’t mean that the genetic material it carries will be damaged; it is usually healthy. According to a 2017 study in Asian journal of andrology, men with 0% normally formed sperm had near-normal fertility rates, which indicates there are other significant factors besides sperm morphology normal.
And men with abnormal sperm morphology can impregnate a female. It means that he is not infertile, but he may need a longer time to make the female pregnant. Which means abnormal sperm morphology and pregnancy is possible. If natural conception doesn’t occur, he will have the option of assisted reproductive technology like In vitro fertilisation with ICSI. ICSI is a process, where his sperm can be directly injected into the cytoplasm of an egg, ICSI stands for Intracytoplasmic sperm injection. The advent of ICSI has furthered reduced the significance and perceived need for sperm quality tests because ICSI requires only one sperm to fertilise the egg. ICSI can be done when sperm morphology is less than 2%.
If sperm concentration and sperm motility are normal or high, it may be advisable and reasonable to consider IUI before ICSI-IVF.